della Dott.ssa Alice Pizziconi
Table of contents
- The pharmaceutical industry………………………………………………………………….4
- How it works………………………………………………………………………………………..4
- Pharmaceuticals is the largest R&D investment in the word………… …………….5
- Great progress with precision medicine resulting from synergies between science and technology………………………………………………………………………………………..5
- Pharmaceutical industry in Italy: increasingly committed to Research…………6
- High competitiveness: a unique business mix in Europe and the strength of pharmaceutical specialisations in Italy………………………………………………..….7
- The impact of Covid-19 on pharmaceuticals and the economy…………………..…8
- Vaccines and prevention……………………………………………………10
- Vaccines are crucial for the health and sustainability of the National Health System………………………………………………………..11
- Types of vaccines…………………………………………………….12
- Vaccine regulation: a little bit of historical background……………..12
- The vaccine law………………………………………………………13
- The covid-19 vaccines………………………………………………………14
- The First Steps Toward an mRNA Vaccine…………….……………16
- What they are and how they work……………………….…………..16
- Production and supply of Covid-19 vaccines…………….………….17
- Covid-19 vaccines: development, evaluation, approval and monitoring……………………………………………………………23
- Patents and Regulation: a general overview…………………………25
- Patents and Regulation: the Covid-19 case…………………………..29
- Pharmaceutical inventions and the complex relationship between industrial property rights and health protection………………………37
- Competition in the regulatory and economic landscape of Covid-19 vaccines………………………………………………………………42
- Economic analysis of Covid-19 vaccines …………………………………49
- Covid-19 related profits………………………………………………51
- Covid-19 vaccine companies and the subsidies from governments…………………………………………………………52
- Covid-19 vaccine companies and the profit spending………….……55
- Biopharma’s stock market winners in 2022, the flotations fail and venture investments………………………………………….…..…..57
- The beginning of a new era: next-generation therapeutics, with the contribution of Dr Rino Rappuoli…………………………………………61
- Relevant mRNA vaccine developments: infectious diseases and cancer………………………………………………………………..62
- The regulatory and legislative situation and perspectives, with the contribution of Dr Rino Rappuoli……………………………………65
- The economic highlights of this new technology, with the contribution of Dr Rino Rappuoli………………………………………………….68
- Future challenges and opportunities for the mRNA vaccines, with the contribution of Dr Rino Rappuoli……………………………………70
Conclusions…………………………………………………………………75
References………………………………………………………………….79
- The pharmaceutical industry
One of the largest and most active sectors in the world, the pharmaceutical sector has 1.2 trillion dollars in revenue and an added value of over 500 billion. More than 60% of the world’s added value is produced in Asia and Europe, yet only the USA accounts for 50% of sales. Ten significant multinational corporations with US and European capital account for about one third of worldwide sales.
With a production value of more than 32 billion euros and an added value equivalent to 0.6% of the national GDP, Italy holds a dominant position at the European level.
When compared to other industrial sectors, the Italian pharmaceutical business has higher added value per employee (+118% over the manufacturing average), larger investments per employee (+313%), and a pronounced propensity to export (+246%), according to competitiveness indexes.[1]
- How it works
The development, manufacture, and marketing of pharmaceuticals are under the purview of the pharmaceutical industry. There are distinct and highly specialized stakeholders present in this complicated and articulated supply chain.
Manufacturers and marketing authorization holders are further up the supply chain (MAs). They are the companies that use a variety of production inputs from the most diverse industries to create the medicine, such as mechanics, chemistry, building materials, plastic, and printed matter, but they outsource distribution. Third parties are in charge of the distribution phase. There is a separation between wholesalers and warehouses. The former are merely in charge of the actual distribution to wholesalers on behalf of the pharmaceutical businesses; they do not own the drug they handle. Depositaries frequently hire specialized transporters to handle the transit procedure instead of handling it themselves. Conversely, wholesalers are required to supply pharmaceuticals in a limited amount of time and are the legal owners of the drugs they sell. Because of this, wholesalers set up shop close to facilities that disperse medications to patients (such pharmacies or hospitals) and make investments in automation, innovation, and technology.
The locations where the patient can pick up or purchase the medication are known as dispensing sites. These locations include ASLs and hospitals as well as pharmacies, parapharmacies, and corners in large retail stores (established as a result of the Bersani Decree, which liberalized the marketing of over-the-counter drugs).
- Pharmaceuticals is the largest R&D investment in the world
Pharmaceutical research and development is a wise investment in growth, safety, and health.
Pharmaceutical companies will invest EUR 1,300 billion between 2021 and 2026, 80% of which will go into an open innovation network made up of various players, including companies, public bodies, start-ups, science parks, and clinical centers. According to the European Commission, the pharmaceutical industry leads the world in terms of R&D investment, both in terms of absolute value and as a percentage of total revenue. A fantastic chance for Italy that might result in an increase in both investment and employment.
84 new medicines were approved globally in 2021. The prospect of a cure for patients and increasingly personalized therapy is boosted by this number, which is the greatest in the last 10 years (55 each year on average) and the more than 18,000 goods under development (some of which will become therapies).[2]
Through funding research, one can increase the health and life expectancy of the populace as well as draw in fresh talent and resources for the economic and social advancement of the nation. To strengthen the innovation ecosystem, basic research, clinical trials, patent registration and protection, technology, and digital data transfer require a desirable environment.
- Great progress with precision medicine resulting from synergies between science and technology
The expansion of the product pipeline is a continual innovation process that can take many different forms but is essential to better meeting patient health demands and raising the competitiveness of the nation.
Gene, somatic cell, and tissue engineering therapies are some of the increasingly specialized goods made possible by scientific and technological advancements as well as the evolution of R&D. These advancements have caused a radical paradigm to shift from therapies based on the “one size fits all” theory to precision medicine and eventually to advanced therapies and “next generation biotherapeutics,” which include these therapies.
With exponential acceleration brought on by network innovation and collaborations with businesses in the digital sector, this is a particularly exciting phase of innovation. Firstly, from science, with a better understanding of people’s genetic make-up, then from technology, thanks to the ability to monitor and analyse massive amounts of data using Big Data Analytics techniques.
The life sciences are moving toward “precision health,” a concept that has significant scientific and societal significance since it allows for better illness detection, earlier diagnosis, more focused and efficient treatment, and less side effects.
- Pharmaceutical industry in Italy: increasingly committed to Research
Pharmaceutical businesses spent EUR 1.7 billion in research and development in 2021, accounting for 6% of all investments made in Italy (+3.7% from 2020).
R&D investment growth from 2016 to 2021 was 14%, a trend that produced some very significant outcomes, particularly in some areas of specialization and increasingly as a result of collaborations with public structures. [3] For instance, biotech medications, vaccines, plasma-derived goods, cutting-edge medicines, and orphan medications.
Companies invest more than EUR 700 million annually in clinical research in Italy (42% of the total on biotech drugs and advanced therapies, 32% of the total on rare diseases and 48% on phase 1 and 2 studies), a crucial step in gaining access to therapies. They frequently do this through SSN structures, while also providing opportunities for professional advancement to doctors and researchers, making cutting-edge therapies accessible to patients while covering all associated costs, such as hospitalization and diagnostic tests.
According to ALTEMS, clinical trial investments return a total of three euros to the NHS for every euro invested. Also, the pharmaceutical industry leads the way in Open Innovation and the percentage of businesses that collaborate with universities and public research institutions. Proof of the significance of pharmaceutical company presence for the expansion of the nation’s whole R&D ecosystem.
- High competitiveness: a unique business mix in Europe and the strength of pharmaceutical specialisations in Italy
With a balanced contribution from businesses with Italian capital, which determines 42% of the industrial function, and those with international capital, which depends on 58%, the pharmaceutical sector in Italy is distinguished by a composition that is unique in Europe.
Pharmaceutical firms take the lead among all the internationally financed businesses in Italy in terms of employment, added value, investments, exports, and also generate significant value through induced purchases.
Italy tops the list of the major European nations for the presence of businesses with US, German, French, Swiss, and Japanese capital. In addition, for businesses with UK funding, it serves as a global base for the manufacture of vaccines.
Italian-owned businesses stand out for their expanding investment in production and research as well as their about 75% of total sales made abroad, which is notably greater than the average manufacturing industry’s growth rate of 40%. Overseas sales have more than tripled over the past 15 years (from 3.1 billion in 2007 to 9.2 billion in 2021), not because of delocalization but rather due to the emergence of new markets, which has allowed Italy to increase its R&D and manufacturing presence. Moreover, Italy leads all of Europe in terms of the number of pharmaceutical SMEs.
The pharmaceutical industry’s expansion is also related to the specializations within it, such as biotech drugs, which are seeing increased investment and a sizable pipeline of products under development, increasingly in advanced therapies, which are also the result of partnerships between businesses and other participants in the national innovation ecosystem.
With a strong scientific background and a strong vocation for exports, Italy is a global R&D and production powerhouse for vaccines. This has allowed it to build up a 4-billion-dollar positive external balance in just ten years.
The Contract Development and Manufacturing Organisation (CDMO), commonly referred to as contract manufacturing, is one area in which Italy leads in Europe. Nowadays, the sector accounts for 2.7 billion in production, or 23% of all production in Europe, in partially due to investments.
Plasma-derived products are one of the specializations, made possible by national businesses with a strong international focus and significant businesses with foreign capital that, in total, employ about 2,000 people and make production and research investments that are significantly higher than the manufacturing average.
- The impact of Covid-19 on pharmaceuticals and the economy
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus.[4]
The pharmaceutical industry is one of the rare exceptions in the crisis environment brought on by the Covid-19 pandemic, continuing to exhibit a robust dynamic and promising development prospect.
But, in this situation, the sector’s organizational structure is not impervious to the significant changes that the numerous lockdowns have brought about on a worldwide scale. In fact, the closures that occurred one after another at various times and in various ways in the major geographic areas have brought attention to the interdependencies and fragilities of a sector distinguished by long and articulated value chains.
In managing the pandemic, the pharmaceutical industry faced a number of difficulties, including difficulty providing the demand for protective gear and diagnostic testing facilities. Pharmaceutical businesses heavily rely on materials manufactured in China, where the Coronavirus infectious disease first emerged in December 2019, as China holds the global monopoly on the manufacture of active components (up to 60% of China’s production)[5].
Due to the closure of facilities, particularly in China, where the majority of pharmacological products are produced, the Covid-19 pandemic has had a direct impact on the scarcity of medical supplies, supply chain uncertainty, logistics, and transportation.[6] The real economy was threatened by the difficulties encountered in commercial transactions and the flow of resources[7]. As a result, businesses either charged expensive prices for these supplies or refused to sell them because they had insufficient stock.
Rules designed to stop the COVID-19 outbreak from spreading restricted the capacity to procure raw materials, make, and deliver medications. In developing and poor nations without enough domestic production capabilities, this presented a significant difficulty. These nations are under tremendous pressure to negotiate affordable treatment choices for individuals in need because of their reliance on imports.[8]
Because of the impact this pandemic will have on low- and middle-income countries’ (LMICs’) fragile healthcare systems, they will face particular difficulties. Healthcare systems in LMICs had a difficult time offering high-quality, reasonably priced, and widely accessible care prior to the COVID-19 pandemic. These healthcare systems lacked appropriate funding, qualified healthcare professionals, and medicine was not readily available. There is a gap in the creation of medications for other chronic diseases as a result of giving priority to COVID-19-targeted drugs and equipment.[9]
The impact of the outbreak on the healthcare industry was shown in the escalating death tolls brought on by inadequate medicine supplies, a lack of vaccination therapy alternatives, inadequate hospital treatment capacity, and a lack of quarantine facilities to handle the mounting COVID-19 infections.[10]
The health problem quickly turned into a global economic crisis. These disastrous effects can be explained by two main aspects. First, due to the virus’s exponential pace of transmission and the increased uncertainty about how terrible things could go, consumers, investors, and global trading partners fled for safety in their spending and investments. Second, as the virus spread, it promoted social withdrawal, which closed down financial markets, companies and businesses, and all other kind of activity or event.
Global stock markets lost nearly US$6 trillion in value in one week from February 23 to 28, 2020, according to S&P Dow Jones Indices, as a result of fear and uncertainty as well as a logical estimate that corporations’ profits were expected to be lower owing to the impact of COVID-19. In the same week, the US S&P 500 index lost more than $5 trillion in value, falling from 3,373 to 2,409, the Nikkei index dropped by 29%, from 23,479 to 16,552, while the FTSE 250 index dropped by 41.3%, from 21,866 to 12,830. In addition, the top 10 S&P 500 businesses collectively lost more than $1.4 trillion.[11]
Given that China was the world’s largest manufacturer and exporter and that the Chinese government had ordered the shutdown of important facilities there, the flow of commodities via worldwide supply chains was drastically restricted.
There was widespread agreement among economists that the pandemic would cause a global recession, and that such stay-at-home policies had sown the seeds of recession in industrialized nations.[12]
In March, the International Monetary Fund predicted a worldwide recession at least as severe as the one that occurred during the global financial crisis of 2007–2008, followed by a recovery in 2021.[13]
As of March 2022, data from the World Health Organization indicated that the COVID pandemic had a varied impact on each region. For instance, the European region experienced the greatest COVID spread, accounting for 41% of all confirmed cases and 31% of all COVID-related deaths. The second-largest geographic dispersion was in the Americas, which included 32% of all confirmed cases and 45% of all COVID-related deaths. The Eastern Mediterranean and African regions had the fewest cases that were confirmed, and the Western Pacific and African regions had the fewest fatalities overall.
The pandemic’s enormous scale and, more importantly, the impact it had during its early stages of propagation led many MPs to favor a policy of extended social isolation while denouncing its negative effects on the economy. The difficult decision that governments had to make over whether to rescue the economy before saving the people or to save the people before preserving the economy was reflected in the recession that followed, which many countries faced.
Vaccines are biological compounds that work to protect the body from infectious diseases by triggering an immune response. Vaccinations guarantee the control of target diseases and the reduction of their incidence, up to, in some situations, up to, in some cases, worldwide eradication, if applied consistently and in accordance with approved plans.[14]
Due to their qualities, vaccinations are one of the best tools for sustainable spending because, by avoiding the spread of diseases, they not only contribute significantly to the population’s health but also result in large financial savings.
- Vaccines are crucial for the health and sustainability of the National Health System
The Covid pandemic brought to light the significance of vaccines, which have allowed for the eradication of some diseases and the control of others, lowering mortality rates and saving millions of lives. Due to their ability to prevent the overuse of antibiotics and the emergence of resistant bacteria, vaccines are also a valuable instrument in the global fight against antimicrobial resistance. Due to the fact that they lower the risk of illness and complications, they are especially essential for the protection of those who are chronically unwell.
For instance, according to data from Vaccines Europe, flu vaccination lowers the incidence of stroke by 24%, heart attack events by 50%, and diabetic patient fatalities by 28%. A contribution that prevents 25,000 deaths in Europe each year while also saving 250 million euros by lowering hospital stays and doctor visits. Another example involving Italy demonstrates that, in 18 years of hepatitis B vaccination, our National Health Service saved a total of €580 million.
So, vaccinations are an investment in the SSN’s sustainability in addition to being good for your health. The cost of the disease prevented as a result of vaccination is 1:16 the cost of immunization. The cost-benefit ratio increases to 1:44 when the resources produced by healthier people are also taken into consideration.
The introduction of effective models of sustainable procurement, with procedures that prioritize greater quality, innovation, and therapeutic value, and with clear indications of needs, is important to assure access and availability of dosages in a setting of growing worldwide competition for supplies.
- Types of vaccines
Vaccines can differ depending on whether the antigens at their core are attenuated, allowing the recipient organism to respond to them without being overwhelmed, or inactivated, putting viruses and bacteria through a process that prevents their ability to replicate by blocking their ability to make proteins through heat or chemical treatment.
Another type of vaccines, in terms of organics, are those that use purified immunogens. These vaccines, which are frequently referred to as “unconjugated saccharide” (or “subunit” (split) vaccines) because they only use fragments of the relevant antigens, are concentrated on bacterial or viral substances that, after being purified and detoxified through modification of the pathogens’ polysaccharide capsule or their protein structure, can trigger a protective immune. Contrarily, conjugated vaccines are made up of many microorganisms that, when combined, produce immunogenic properties that were not there before. The last option is anatoxin vaccines, which are made from molecules from the infectious agent and are adequate to activate the body’s immune defences despite not being able to cause disease.[15]
- Vaccine regulation: a little bit of historical background
Early in the 19th century, as smallpox vaccination swept throughout Europe, the practice of requiring vaccinations became widespread. In reality, doctors had observed that by safeguarding the individual, it was possible to stop the pandemic from spreading to the entire community, but they had also noted that this required extremely high levels of commitment. Together with enthusiasm, the introduction of vaccination sparked strong opposition.
According to a school of thought that originated in Germany, the state must actively take care of keeping its subjects in the best possible state of health in order to obtain healthy and numerous soldiers and taxpayers, the decision to intervene in a mandatory and organized manner to protect public health was part of that school of thought. Smallpox vaccination was the first coercive measure to be spread throughout Europe, and it didn’t take long for it to encounter violent opposition in England, the birthplace of liberal doctrines opposed to anything that might come from the power of government and interfere with citizens’ freedom of choice.
Due to its great scientific contributions, its forward-thinking vaccination laws, the knowledge of its researchers and public health professionals, and even some industrial vaccine production, Italy has long been a leader in contemporary healthcare.
Four phases have been recognized regarding the development of vaccination policies in Italy over the first 40 years of the National Health Service. The first period (1978–1988) was marked by the eradication of smallpox, hopes for further eradication, and the introduction of the hepatitis B and acellular pertussis vaccines. A comparatively small number of vaccines were available during the initial phase of the universalist health reform of 1978, with safety goals taking a backseat to protective efficacy. The second period (1999–2008) was characterized by the introduction of the first national vaccination plans and the hypothesis of a gradual shift from mandatory to conscious adherence, marked by the significant experimentation of the Veneto Region. The third phase (2009–2014) was marked by the expansion of health information on the internet and social media, anti-scientific legal judgements, and an increase in vaccine hesitancy that resulted in misconceptions, generalized coverage declines, and the resurgence of epidemic outbreaks; The institutions responded during the most recent period (2015–18), which resulted in the PNPV 2017–19 being approved, the extension of vaccination requirements, and punishments against antivaccination doctors. This sparked a frenzied political and media debate about the moral and ethical implications of punishments and restrictions on the entrance of unvaccinated children to schools, as well as a sharp increase in coverage.[16]
- The vaccine law
The Vaccination Decree increased from four to 10 the number of mandatory vaccines for children and adolescents in our nation. The goal is to reverse the gradual reduction in vaccination rates—both required and recommended—that has been occurring since 2013 and has caused our nation’s average vaccine coverage to fall below 95%. The World Health Organization has suggested this level to ensure “herd immunity,” or the indirect protection of even individuals who cannot receive a vaccination due to health concerns. As stated in the agreement signed by the Permanent Conference for Relations between the State, the Regions and the Autonomous Provinces of Trento and Bolzano on January 19, 2017, it will also enable the achievement of the National Vaccine Prevention Plan’s top priorities for 2017 to 2019 as well as compliance with obligations made at the European and global levels.
Conversion Law No. 119 of July 2017, which revised Decree Law No. 73 of June 7, 2017, Requiring measures on vaccine prevention, effectively mandates vaccinations for children between the ages of zero and sixteen as well as unaccompanied foreign kids.[17][18]
- The covid-19 vaccines
Since the genetic code of the SARS-CoV-2 virus was revealed on January 11, 2020, scientists, business, and other organizations have worked together to create COVID-19 vaccinations as quickly as feasible.
Some vaccines are created utilizing the same technology (or “platform”) as those that are now in use, while others are created using fresh ideas or ideas that were recently employed in the creation of the SARS and Ebola vaccines. All of these vaccinations aim to trigger an immune response that will neutralize the virus and stop cell infection.
- Inactivated viral vaccines: the SARS-CoV-2 virus is cultured in cell cultures and chemically inactivated
- Living attenuated vaccines are created by creating a genetically weakened version of the virus that only partially replicates, does not spread disease, but instead triggers immune reactions like to those triggered by a genuine infection
- Recombinant protein vaccines: based on the spike protein, or receptor binding domain (RBD) or virus-like particles (VLPs)
- Viral vector vaccines: typically based on an existing virus carrying the genetic information for the spike protein (normally an adenovirus incapable of replicating)
- DNA vaccines: plasmid-based constructs that have been altered to carry genes that code for the spike protein, which the person receiving the vaccination subsequently produces.
- RNA vaccines: These vaccines are built on messenger RNA (mRNA), or self-replicating RNA, which contains the genetic code for the spike protein.[19]
One of the main problems associated with the development of these new vaccines is the controversial regulatory and intellectual property debate, which is already complex in the general pharmaceutical context, but even more so in situations such as the terrible Covid-19 pandemic that hit the world in December 2019. This global health crisis brought to light obvious issues related to the regulation of vaccines and their production, setting the stage for the start of an ethical and scientific debate that continues to this day.
Since the WTO’s inception (1994), each nation has the power to, under special conditions, limit the influence of pharmaceutical companies on its own soil by requiring them to permit the production of a protected drug that is deemed urgent by one or more third parties. It is a system known as “compulsory” licensing, which implies that the nation seeking to utilize it must pay the patent holder compensation. This exception was expanded by agreements reached in Doha in 2001 and formalized in 2005, which permit individual countries to issue mandatory licenses for the production of generic medications as well as the import and export of those drugs in support of other nations who are in need but unable to produce them. The European Union established a law governing the use of this instrument in the event of a health emergency in 2006. This was done specifically so that they would be prepared to respond to this kind of request.
The core principle of a patent is the transfer of technology from a private party (the inventor) to a public authority, which in each country where legal protection is sought is the patent office. In the pharmaceutical industry, this means that a company in the same field, with the necessary technical know-how, technology, and materials, should be able to reproduce it by reading the “recipe” of a vaccine, and consequently the composition and production methods of the active ingredient, as described in the application filed with a patent office. If it doesn’t, patents for vaccines against Covid-19 might not be issued on the legal presumption that not enough knowledge has been transferred and that too much production-related information (know-how) is still kept a secret.
It is crucial to emphasize that present rules give pharmaceutical corporations additional exclusive right over their products, which is treated like a trade secret, in addition to the patent. As a matter of fact, businesses are given the only right to the clinical trials and test results required for the development of a new drug, in accordance with safety regulations examined by organizations like the European Medicines Agency (EMA). Although extremely comparable to intellectual property rights, this exclusivity forbids the development of a generic before the elapse of a term that, under EU legislation, lasts between 8 and 10 years starting from the moment of marketing. It appears quite clear that without access to testing and clinical studies that would enable quick approval for manufacture, even if one or more governments were to restrict or temporarily stop vaccine patents, these steps would be insufficient for the creation of generics.
- The First Steps Toward an mRNA Vaccine
In place of conventional vaccine techniques, nucleic acid therapies have shown promising. In vitro transcribed (IVT) mRNA was successfully used in animals for the first time in a research that was published in 1990, when scientists learned that they could inject mice with mRNA and DNA to induce the production of a protein. For a few weeks, that protein production continued. In 1992, diabetes insipidus symptoms were cured in rats by administering mRNA coding for vasopressin (anti-diuretic hormone). These results led to the hypothesis that an animal’s cells may produce a viral or bacterial protein, which the animal’s immune system would subsequently fight off. The instability of mRNA itself was the only thing getting in the way. It simply dislikes being outside of cells.[20]
The race to find a means to transfer mRNA without it becoming unstable lasted from the 1990s to the 2010s. At that period, advancements were made in the development of vaccinations against cancer, allergies, and parasites. Many businesses were working on mRNA vaccines with somewhat stable delivery mechanisms by the time the coronavirus pandemic hit. They were able to undertake extensive clinical trials almost simultaneously with optimizing their formulations thanks to the urgency of the epidemic and the government financing they obtained.[21]
- What they are and how they work
In the traditional vaccinations, the “weakened” virus (or bacteria), or a portion of it, is typically injected. The immune system detects the “intruder” and creates antibodies it will employ to fight the “actual” invader. On the other hand, in the case of RNA vaccines, the “instruction” is administered to generate a specific protein known as the “spike” protein, which the virus utilizes to “attach” itself to the cells. The “foreign” protein is then created by the cell itself, and once it is recognized, it causes the body to make antibodies.
In 1961, messenger RNA was identified. It is essential for the production of proteins, which is essential to human existence.[22]
Although the DNA does serve as a sort of “storage” for the recipe for making proteins, messenger RNA is ultimately responsible for spreading it throughout the cells and transmitting information about how and when to make proteins.
Hence, messenger RNA functions as a kind of postman who delivers significant signals to the cells. As a result, the concept of injecting information, messenger RNA, into cells to make a therapeutic protein, which was first proposed in the 1990s, was born.
RNA is a transient, delicate molecule that only exists in cells when it is performing a specific purpose and degrades rapidly. The danger is that it could be quickly “demolished” before the message even reaches the cells.[23]
By encasing the fragile RNA molecules inside little bubbles of fat called RNA-lipid nanoparticles (LNP), which were made possible by the development of nanotechnology, they are able to go to their destination unharmed. The messenger RNA molecules are released inside the cell itself as a result of the fat layer fusing with the cell’s outer membrane. The mRNA that includes the instructions required to make the virus’ spike protein is released by the liposomes after they are injected into our bodies. Ribosomes are found in every one of our cells and are responsible for turning mRNA data into proteins. Once the vaccine’s mRNA has entered the cells, the ribosomes will decode it and manufacture several copies of the virus’s Spike protein. [24]
The Spike protein will leave the cell after being produced, at which point the immune system will identify it as foreign. It is crucial to emphasize that, although causing an immune response, the Spike protein alone cannot cause disease because it only makes up a small portion of the virus.
The immune system is now in the process of working. It begins making memory cells and antibodies against the Spike protein of the virus. The Spike protein will be blocked by the antibodies, preventing the virus from spreading. The body’s memory cells will continue to exist and act as a form of defense throughout time.[25]
- Production and supply of Covid-19 vaccines
On January 11, 2020, the genetic code of SARS-CoV-2, the coronavirus that causes COVID-19, was made public. This sparked a surge in global R&D efforts to create a vaccine to prevent the illness. The first COVID-19 vaccine candidate entered human clinical testing with unprecedented speed on March 16, 2020, as a result of an evaluation of next-generation vaccine technology platforms using novel paradigms to speed up development. This was made possible by the scope of the COVID-19 pandemic’s humanitarian and economic impacts.
The difficulty lay in producing the vaccinations in sufficient quantity to give the world’s population immunity as soon as possible. Indeed, the challenges of figuring out how to expand the manufacturing of vaccine doses to combat the pandemic have been made more difficult by how quickly safe and effective vaccines have been created and approved for emergency use. The amount of time necessary for such learning to take place has been severely constrained. Even though there was no guarantee that any of the vaccine candidates would ultimately be licensed for use, vaccine developers and government and civil society organizations concerned with the COVID situation had to create plans for the mass manufacture of vaccine.[26]
Achieving access to manufacturing facilities, obtaining raw materials for the manufacturing process, and scaling up production to achieve high levels of efficiency have all been necessary for the mass manufacture of the vaccines. The developers started working with one or more manufacturing partners as soon as a vaccine candidates entered the first stage of clinical trials to expand the capacity to mass produce at the fastest attainable rate. The fact that the vaccines now approved for emergency use in various nations were produced utilizing cutting-edge technologies complicates the difficulty of mass producing COVID vaccines. Among other benefits, the creation of mRNA and recombinant-vector vaccines has made it possible to find potent vaccine candidates and move them quickly toward clinical trials. The manufacturers have little past knowledge on the scaling of the process because the technology platforms that have been employed to generate the COVID vaccines are novel.
The COVID vaccinations that have been created so far are given intravenously. Scaling up COVID vaccines faces unique difficulties due to the requirement for injectables in large quantities.[27] According to the diagram below, there are three main steps in the COVID vaccine manufacturing process:
- design of the pre-manufacturing process and facility setup;
- manufacture of drug substance (DS), which consists of the vaccine’s active pharmaceutical ingredients (API), which work together to produce antibodies that block viral infections from attacking human cells by neutralizing their effects;
- the process of creating actual drug products (DP), which involves placing bulk DS into syringes or vials that have already been filled. There are a large number of quality control tests spread throughout DS and DP.
Stages of the vaccine-manufacturing process
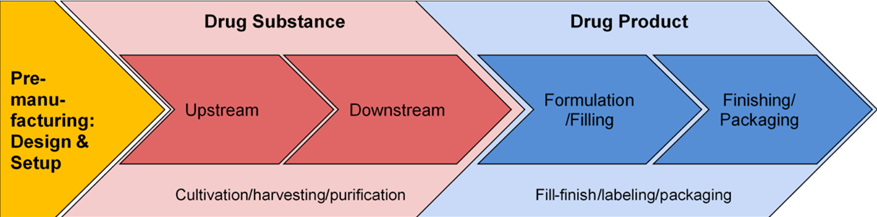
Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: What manufacturing activities are involved and why are they so difficult? 2021
The two manufacturing stages—DS and DP—involve varying degrees of difficulty depending on the vaccine technology utilized.
The primary production of mRNA vaccines depends critically on an ample supply of lipids. The specialist markets that require lipids are only served by five large producers globally, and these lipid suppliers have their own scaling issues.[28]
Increasing production of previously specialist substances, such as lipid nanoparticles, for a global vaccine drive has been one of the most difficult tasks in recent years. The ionizable cationic lipid (ICL), one of the four lipids that make up the protective droplet in mRNA vaccines, is the one that requires the greatest volume and is covered by limiting patents held by Acuitas Therapeutics, a tiny biotechnology firm in Vancouver, B.C. and a few other businesses.
Additionally, PEGylated Phospholipids (PPLs) are fairly rare. As excipients in COVID vaccines that contain viral-vectored and mRNA, functional lipids are making their vaccination debut. However, PEGylated Lipids have been used therapeutically since 1995, when the US Food and Drug Administration approved the first nanodrug. [29]
The creation of high-quality lipids is the last step of a process that takes around two dozen steps and involves several purification stages to create synthetic ICLs. Only a few suppliers are available globally to complete such a difficult production procedure, with the top three, so 14% of all lipid suppliers, accounting for almost 90% of all citations for lipids worldwide.
Dominating in 2017 with a gigantic 70% share, MilliporeSigma was at the top of the charts for the supply of lipids worldwide. As lipidomics research develops, the company plans to add to its line of more than 1700 lipid products. Avanti Polar Lipids, established in 1967 by Walter A. Shaw, was the second-largest provider of lipid products globally. With a portfolio of more than 2000 distinctive lipid products, Avanti Polar Lipids possessed a 17% share of the global market.[30]
As we previously stated, only a few numbers of businesses worldwide genuinely possess the tools and infrastructure necessary to produce lipid nanoparticles or the unique cationic ionizable lipids. Only a handful of other companies have equipment and facilities that can be upgraded to produce more. For Pieter Cullis, biochemistry professor, considered the “grandfather” of the lipid nanoparticle technology, and co-founder of the company Acuitas Therapeutics, “the holdup seems to be more on the manufacturing of the other components like the ionizable cationic liquid and cholesterol, which are two of the larger components of the lipid nanoparticle.”
Lipid suppliers are unable to meet the pandemic-driven worldwide lipid demand, even after the vaccine producers plan for the retooling of new facilities to enhance production. In addition, the recruitment and training of highly qualified scientific people capable of increasing output while preserving the quality of the lipids has been a key challenge for lipid manufacturers.[31]
Working with other businesses that can upgrade their facilities and increase capacity to make lipid nanoparticles has proven to be the most effective option for vaccine manufacturers to handle these supply chain concerns.
Pfizer, for example, pledged to increase its capacity for producing lipids while simultaneously purchasing lipids from Croda, a British chemical business, and Avanti Polar Lipids, Croda’s Alabama-based subsidiary. Contracts for the Pfizer/BioNTech vaccine also exist with Merck KGaA and Evonik, both of which are situated in Germany. The CEOs of Pfizer and BioNTech formed also a unique agreement with the lipid supplier Polymun in September 2020 to transfer its lipid formulation know-how to Pfizer facilities after signing supply contracts with nearly all major lipid producers. Pfizer was then able to begin producing lipids at its global R&D headquarters in Groton, Connecticut, in March 2021 and raise its projection for worldwide COVID-vaccine production for 2021 by directly controlling the manufacturing of lipids.[32] Then Johnson & Johnson, whose vaccines were produced in collaboration with US-based Merck & Co. Finally, to rise its supply of lipids, Moderna increased its collaboration with CordenPharma, which produces lipids in both Europe and Colorado.
The mRNA vaccine manufacturers have had to learn how to create encapsulated vaccines, which requires the rapid and extensive mixing of mRNA with lipids, even after they have solved the lipid supply problem. The impingement jet mixer (IJM), which is a crucial piece of machinery for this procedure, prepares medication items that can later be filled into vials or syringes and shipped to procurers. The most crucial step in the manufacture of vaccines is the encapsulating of mRNA with LNPs, which requires high levels of accuracy. In order to address the rising demand for mRNA vaccines, it is also necessary to improve the microfluidics mixing solutions already on the market. The fact that there are now just two main IJM providers in the world who are able to upgrade the technology for microfluid and nanofluid mixers further complicates the situation.[33]
After completing the production of vaccines, they are subjected to three distinct phases of clinical development and tested on voluntarily participating patients in clinical trials. The goals are to confirm the vaccine’s safety and to prove the vaccine’s effectiveness. Dose-toxicity studies are a part of the early-phase trials, which help researchers discover how well participants tolerate various dosage levels. The vaccine candidate with the best safety profile is chosen by researchers for the phase III double-blind, placebo-controlled efficacy trials based on the findings of these studies. The move from small-scale vaccine manufacture for clinical trials to the bulk manufacturing of doses for commercial use was significantly impacted by the extreme brevity with which each company’s scientists had to choose its candidate for phase III testing.[34]
The logistics of vaccine distribution for later administration and the expense of cold-chain storage are typically the biggest problems faced by the worldwide vaccination campaigns. Over time, vaccines gradually lose their effectiveness, but high temperatures hasten this process. In order to ensure vaccine stability and immunogenicity, which are diminished even at moderate temperatures, vaccines are often maintained at cold or ultracold temperatures. According to the vaccine platform, different vaccines have different heat sensitivity, and even vaccines developed using the same platform can have different stability profiles depending on the manufacturer.[35]
From the moment of vaccine production until the time of delivery, or just a few hours prior, the cold chain should be maintained. It is the duty of vaccine producers, distributors, public health officials, and healthcare professionals to maintain the cold chain. Three essential components—well-trained staff, dependable storage and temperature monitoring equipment, and precise management of vaccine inventory—make up an effective cold chain. When a vaccine is exposed to an unsuitable environment, such as inadvertent warming or freezing owing to a break in the cold chain, its effectiveness may be reduced and cannot be recovered. A single exposure to a freezing temperature can cause any refrigerated vaccine or diluent to become ineffective. When rapid mass vaccination is required, populations who mistakenly received vaccines that were exposed to unsuitable temperatures should be revaccinated. This requires more doses for the patient and additional expenses for the provider. In particular in climatic zones (i.e., 30°C/65% relative humidity), the need for ultracold temperatures for the storage and transport of the most advanced COVID-19 mRNA vaccines, especially the 70°C freezing condition required by some of them, is a major barrier to vaccine distribution and thus rapid mass immunization. To preserve their quality and effectiveness, these vaccinations should be delivered and maintained in a carefully monitored environment. The equipment for storage and transportation should adhere to WHO standards but it is expensive to purchase.[36]
Depending on the specific formulation of mRNA within LNPs, as well as the vaccine’s lipid and non-lipid components, the ideal temperature level for a vaccine to be stable is determined. When the lipid components utilized in their synthesis fail to bond together, LNPs can become extremely unstable. Physical degradation is another important factor in LNP stability. Aggregation, fusion, and leakage of the pharmaceutical substance that is encapsulated are the three basic categories of physical instability that might happen. The best way to keep a vaccine safe is to keep it at a very low temperature, which varies depending on the formulation of the specific vaccination. It is essential to have instruments to monitor these factors since the integrity and purity of the mRNA are crucial for preserving the effectiveness and safety of mRNA vaccines.[37]
All of the excipients in a vaccine that will be used in clinical trials must be approved by the Food and Drug Administration (FDA). Therefore, several makers created LNPs utilizing lipid components that were already known to be safe in order to minimize regulatory delays in starting clinical trials as well as unanticipated toxicity difficulties.
3.4. Covid-19 vaccines: development, evaluation, approval and monitoring
A significant part of enabling the development, scientific review, approval, and oversight of COVID-19 vaccines in the European Union is played by the European Medicines Agency (EMA) (EU).
The COVID-19 vaccines are created, examined, and approved in accordance with the most recent scientific findings as well as any relevant statutory requirements.
An application incorporating information from multiple studies must be submitted to EMA by a company developing a COVID-19 vaccine. Firstly, studies on pharmaceutical quality reveal information regarding the quality of the vaccine. This covers the vaccine’s active ingredients, purity, and other substances (such as stabilizers), as well as its manufacturing and control processes, stability, and shelf life, and the best ways to store it. Secondly, non-clinical or laboratory investigations that indicate if the vaccine might result in safety issues, which in extreme circumstances could include effects on development or reproduction. Ultimately, human clinical studies that demonstrate a vaccine’s safety and effectiveness.
These trials must examine immunological responses, effectiveness, and safety for COVID-19 vaccines.[38]
The EMA’s recommendations for COVID-19 vaccinations that have been modified are based on all available information, including fresh data for the adapted vaccines and a substantial body of prior research for the COVID-19 vaccines that were initially authorized.
A firm creating a COVID-19 vaccine must also give thorough justifications for the usage of each vaccine component and the manufacturing process it employs.
The COVID-19 vaccinations are initially examined in a lab, then, in experiments known as clinical trials, vaccines are tested on volunteers who are humans. These tests support how the vaccinations function and, more crucially, assist determine their safety and level of protection. Companies can request permission from the European Medicines Agency to market the vaccine in the EU once there is enough information from studies and clinical trials. The European Medicines Agency analyzes all the information and performs a thorough and impartial scientific evaluation of the vaccine. Finally, the European Commission provides an EU marketing authorization based on the scientific evaluation of the Agency. Thereafter, the vaccination can be applied.[39]
Indicative timelines for COVID-19 vaccines compared with standard vaccines:[40]
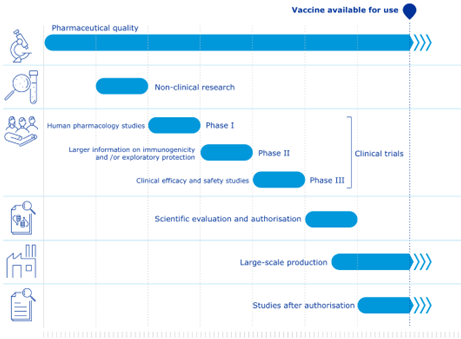
Figure 1 Standard Vaccines
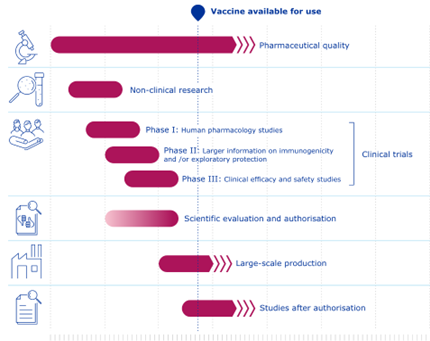
Figure 2 Covid-19
The pharmaceutical laws of the European Union (EU) make sure that vaccinations are only authorized when a scientific analysis has shown that their overall benefits outweigh their hazards. The advantages of a vaccination in preventing COVID-19 must outweigh any negative effects or potential hazards by a significant margin.
The European Medicines Agency (EMA) makes ensuring that scientists who evaluate drugs are free from financial or other conflicts of interest that can skew their judgment. See Managing opposing interests for further details. The independence of EMA’s scientific evaluations is protected by a high level of transparency, which makes EMA’s scientific evaluation work available for public review. See Transparency: Special Measures for COVID-19 Drugs for further details.
According to the EU pharmaceutical legislation, the usual evaluation period for a drug is up to 210 active days. Nonetheless, EMA handles requests for COVID-19 product marketing authorizations quickly. This enables the review period to be shortened to less than 150 working days. The EMA may employ its COVID-19 rolling review process for potential medications in this context. This enables EMA to evaluate data as they become available throughout the development phase, speeding up the evaluation of the ensuing formal marketing authorisation application.[41]
3.5. Patents and Regulation: a general overview
Pharmaceutical inventions’ patentability has always been a contentious topic of discussion.
Some vaccine technology components are not covered by intellectual property laws. The most notable instance may be the standard formulation for a number of vaccinations that have been around for a while but are no longer protected by patents.
On the other hand, the majority of recently created vaccinations have many parts that can be protected by one or more patents and are frequently so. The era of innovative vaccination R&D free from restrictions imposed by intellectual property laws has been replaced by one where patents are valued highly. [42]
Focusing on the Italian patent in the pharmaceutical industry, it was legitimated by the Constitutional Court only forty years ago (Corte Cost., sent., 20/1978), making it far from a legislative effort. As a result, the original legislative prohibition, which dates back to the reign of Victor Emmanuel II, underwent a revolutionary jurisprudential decision based on a profoundly negative impression of the institution, which was perceived as a tool for monopolistic speculation and a barrier to study.
The Piedmont law of March 12, 1855, No. 782 is where the prohibition against patenting medicines first appeared, and it was finally codified in Article 14, c. 1 of the so-called Inventions Law (Royal Decree June 29, 1939, No. 1127).
The Constitutional Court ruled that Article 14, c. 1., l.i., was unconstitutional because it violated certain constitutional standards (Articles 3, 9, 41, 42, and 43 Const.), marking a turning point in Italian history that saw the principle of drug patentability finally accepted into the country’s legal system.
In addition to the stark contrast with some constitutionally guaranteed values, such as, for example, research and the protection of public health, of which, precisely, research and patenting appear as its instrumental elements, the Court claimed that the reasons that had led the Parliamentary Commission to deny the patentability of drugs years earlier could no longer be considered in line with the current reality.
Given the disparity it created between operators in that field and those in other fields of technology, upholding an absolute ban on patenting in the pharmaceutical field would have disincentivised companies from investing in research and developing a drug, with foreseeable repercussions on the level of competition as well.[43]
The Supreme Court’s decision has historical resonance even today, not only because of the outcome reached after nearly 127 years of supremacy and resistance to the ban on drug patenting, but also because it highlighted the problematic relationship between the use of patents, competition regulation, and health protection, which has recently resurfaced to a significant degree in relation to vaccines.
In the emergency context, the problematic intersection of patents, medications, and competition seems to have resurfaced to a major level, with pharmaceutical firms as the protagonists.
The topic of pharmaceutical patents has never been as important as it is at the historical moment marked by the Covid-19 pandemic, particularly when it comes to the issue of vaccines, the scope of which, from a competitive point of view, lends itself to dynamics quite different from those defining the pharmaceutical sector due to the complexity of the patents and the complete absence of a generic version in the vaccine industry.[44]
Most people have developed a strong sense of rejection with regard to intellectual property rights as a result of the health and socioeconomic crisis, which has served as fertile ground for the reemergence of issues and uncertainties and exacerbated the negative perception of the colossus of Big Pharma (Pfizer, Roche, AstraZeneca, Johnson&Johnson, Novartis…). Based on these presumptions, many scenarios and the potential influence of the patent institution were extensively addressed, but the main focus was on how to be able to balance the competing interests in a way that is both essential and desirable.
There have been a variety of proposals and points of view on the matter, ranging from the use of the instrument of compulsory licensing mentioned in Articles 70 and following of the Industrial Property Code and Article 33 of the TRIPs Agreement to the expropriation of patent rights for public utility, all the way up to a suspension or complete abolition of the patent as proposed by Asia and South Africa in October 2020 and referred to by some as “dead-end streets“.[45]
The attitude adopted by some of the top pharmaceutical corporations, for which analytical authoritative doctrine is referred to elsewhere, is one of the many elements that are not taken into account.[46]
Regarding the position taken by our government, direct legislative action was taken, following the approval by the Houses of Parliament of the amendment sought by the former Minister of Health to the so-called “D.l. Recovery” (Decree Law No. May 31, 2021, no. 77, Governance of the National Recovery and Resilience Plan and Initial Measures to Strengthen Administrative Structures and Accelerate and Streamline Procedures), novating the Industrial Property Code by introducing a new provision – Article 70-bis – bearing the possibility of resorting to the instrument of compulsory licensing for drugs and vaccines in the event of a health emergency.
Thus, the government was given the authority to require patent holders of drugs and vaccines to grant their non-exclusive use to the state or third parties, subject to a few requirements: that the licensing company receives adequate compensation; that the drugs and vaccines are “essential” to deal with the emergency; and that there is a “proven difficulty of supply.” This is, of course, a temporary concession, lasting only as long as the emergency lasts and no longer than twelve months after it ends.
The Marrakesh Agreement, which led to the creation of the World Trade Organization on January 1, 1995, is a milestone in the history of international intellectual property protection. It was the biggest change to global trade since the end of World War II.
The two primary responsibilities of the WTO are outlined in Article III of the Marrakesh Agreement: 1) a forum for negotiations over rules governing international trade; 2) a body for the resolution of problems involving international trade.
The fundamental premise that the protection of intellectual property rights advances technological innovation and makes it easier to transfer and disseminate technological information for the “mutual benefit of producers and users” of technological knowledge is the basis for the TRIPs understanding.
In order to better strike a balance between the legitimate interests of rights holders and users, the framework additionally stipulates that the exclusive rights granted may be subject to restrictions and exceptions.
“In exceptional circumstances, the Ministerial Conference may decide to grant a waiver of an obligation imposed on a Member by this Agreement or by a Multilateral Trade Agreement, provided that such a decision is taken by three-fourths of the Members, unless otherwise provided (…)”. This is stated in Article IX, “Decision-making Process,” para. 3 of the Marrakesh Agreement. In addition, Paragraph 4 mandates that the extenuating circumstances supporting the choice, the rules for how the waiver will be used, and the waiver’s expiration date all be made clear.
The exceptions system that permits the exclusive rights of patent holders to be waived is more specifically regulated by the TRIPs Agreement. They can be divided into two categories: general exclusions (Article 30 TRIPs Agreement) and flexibilities available to WTO member states (Article 31 TRIPs Agreement).
The requirement in Article 31(h) of the TRIPS Agreement that the patent holder for whom a “other use” is approved receive appropriate compensation that takes into account the economic worth of the authorisation may be disregarded in accordance with Article 31bis. The European Union implemented Regulation (EC) No. 816/200620 in accordance with Article 31bis of the TRIPS Agreement, which regulates the granting of compulsory licenses for patents and supplementary protection certificates (SPCs) for the production and sale of pharmaceutical products intended for export to states that require them for public health needs.
As part of the proposed initiatives for the “suspension” of patent protection, the governments of India and South Africa sent a joint proposal to the World Trade Organization on October 2, 2020, asking for a derogation from the terms of Article IX, clauses 3 and 4, of the Marrakesh Agreement with regard to patents and other intellectual property rights related to vaccines, drugs, and other medical technologies for the duration of the pandemic, until the point at which the pandemic has been eradicated.
The TRIPS Council has discussed the initiative of India and South Africa in several meetings, but finally, the World Trade Organization, during a Ministerial Conference, Twelfth Session, in Geneva, June 12-15, 2022, released a document that also covered the topic of Covid vaccines and other intellectual property measures related to vaccines and medical tools useful in containing and combating Covid 19 infection:
“Notwithstanding the provision of patent rights under its domestic legislation, an eligible Member[47]may limit the rights provided for under Article 28.1 of the TRIPS Agreement (hereinafter “the Agreement”) by authorizing the use of the subject matter of a patent[48]required for the production and supply of COVID-19 vaccines without the consent of the right holder to the extent necessary to address the COVID-19 pandemic”.
The World Trade Organization (WTO) document, which was endorsed by 164 member countries, permits the “waiving of certain requirements regarding the compulsory licensing of COVID-19 vaccines,” though the text makes clear that the measure only lasts for five years and is only intended for certain nations.
3.6. Patents and Regulation: the Covid-19 case
Since the introduction of the initial SARS-CoV-2 vaccinations, often known as Covid vaccines, in several countries, the challenge has been to both assure their accessibility to the world’s poorest nations, for whom market prices are unaffordable, to secure their availability through domestic production, to prevent undue demands on the public purse, and to avoid unnecessary burdens. For this reason, expropriations and other authoritative measures have been suggested.
At the time, neither known patent applications nor particular patents on vaccinations against SARS-CoV-2 could possibly exist. Actually, the SARS-CoV-2 virus sequence was originally reported on 10.1.2020, making that date the official date the virus became known. No application for a patent on a SARS-CoV-2 vaccine may be accessed before July 2021 due to the 18 months of confidentiality. And this is true even in the case that vaccination could be created as soon as the sequence was filed. Only a request for early publication can waive secrecy.[49]
From a purely economic standpoint, it is important to keep in mind that neither under Italian nor international law are compulsory licenses and expropriations free of charge.
Italian law stipulates that the expropriated holder must be compensated “on the basis of the market value” (CPI, art. 142). As the Constitutional Court has repeatedly stated in cases involving real estate expropriations, “on the basis” does not always imply full value, but one could not go significantly below it either. It is simply not conceivable to expropriate a patent for free or to pay substantially less for it than its market worth, since the applicable patent’s value for a Covid vaccination would surely be quite high.
The same holds true for mandatory licensing. The Covid outbreak led to the introduction of a rule-specific regulation for health emergencies that offers the holder an “appropriate remuneration… determined taking into account the economic value of the licence” (CPI, Art. 70a). Additionally, the compensation was not small in the few compulsory licenses that were previously given (although in different circumstances).
But there is a more significant issue that makes drastic measures like expropriations, mandatory licenses, and other similar practically impossible. In reality, these actions must be ordered in respect to particular patents; in other words, any expropriation, compulsory license, or other step must expressly state which patent(s) it refers to. This would only be possible in the case of a vaccination if its patent coverage was evident or at the very least simple to verify. However, this is typically not the case with vaccinations, and it certainly isn’t the case for Covid vaccines, for which the patent situation is complicated.
We should keep in mind that each vaccine is made up of numerous parts and is the result of a vast amount of knowledge that has been developed over time and even by different subjects, each of whom has a portfolio of patents that may be relevant to the vaccine itself and thus require licenses.
As a result, each vaccination may not only be covered by one or more patents owned by the firm that developed it, but also by several licenses on patents owned by other businesses. Patents that don’t always directly claim a vaccine because they can cover things like individual parts or different ways to deliver or stabilize the vaccine themselves (which complicates vaccine patent searches).
The so-called Spike protein is the basis for the Covid vaccines in particular, or at least the ones that have received the most attention. It is a feature of the virus that enables entry of the virus into the cell itself as well as attachment and fusion with the cell. The Spike protein, or more specifically, a portion of it, is the antigen, or the substance that the immune system recognizes, and which prompts an immunological response. The Spike protein is triggered in diverse ways in the numerous vaccinations.
Each of these vaccines has a wealth of knowledge that has been accumulated over many years and has gradually allowed the solutions to an equal number of difficulties. Many solutions are covered by patents owned by parties other than the developing company, which necessitates licenses and occasionally legal action.
In very general terms, it should already be recalled that because mRNA is so unstable, it must be enclosed in tiny fat particles known as lipid nanoparticles (LNPs), which have been extensively studied for decades and are the subject of a large number of patents from companies that are either competing manufacturers or wholly unrelated to the production of vaccines. Additionally, modified forms of mRNA must be employed, which are also trademarked by various firms, in order to preserve it against degradation. Finally, a mutation that codes for a Spike protein with a conformation that prevents the virus from fusing into the cell is a very significant change. In connection with this, there are significant patents that vaccine producers must deal with.
Each mRNA vaccine in such a situation is found to be protected by numerous other patents, which means that producing it may require numerous licenses and cross-licences, many of which are not available to the general public. The scenario, which is so complex that it was defined as a “patent maze” by Ulrich Storz (The patent maze of COVID 19 vaccines, 2021) was previously visualized as follows:
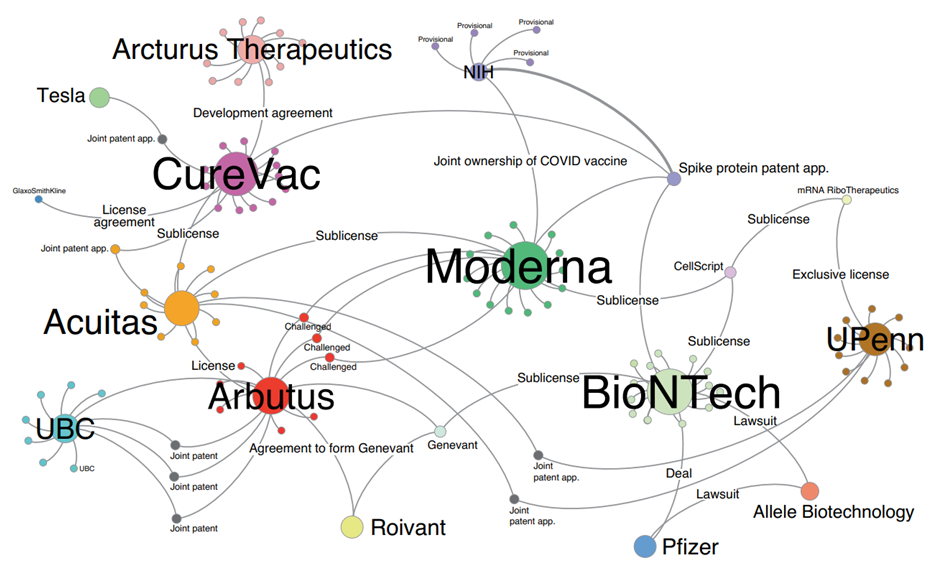
Fig. from Patent network analysis of mRNA-based vaccine candidates for COVID-19.[50]
The situation is just as complex for vaccinations other than mRNA vaccines.
Going back to the beginning, it is evident that it would be virtually impossible in such a circumstance to identify the pertinent patents for the purposes of potential expropriation or coercive licensing. Without leaving out the fact that it would be required to be antagonistic and to arrange recompense with each holder. This makes authoritative interventions of this kind seem unworkable.
Evidently aware of the situation, some nations had suggested an intervention of a completely different kind inside the framework of the World Health Organization. The proposal called for a “waiver” that would have released member states from the requirement to uphold specific TRIPS agreement provisions “in relation to the prevention, containment and treatment of Covid-19” for a predetermined period of years, in place of expropriations or compulsory licenses on specific patents.[51] These requirements were included in Part II’s Articles 1, 4, 5, and 7, which dealt with copyright, design patents, and secret information. This meant that any patent, know-how, or other protection would be invalid if it had anything to do with preventing, containing, or treating Covid. And all of this happens automatically, wherever in the world, without the need for any special precautions. However, a less comprehensive choice, the scope of which we previously discussed, ultimately replaced this suggestion.[52]
The World Trade Organizations’ final decision has never actually been put into reality (Article 5 states that in order to use it, a state must first notify the Council of TRIPS, which hasn’t yet received any notifications).
One issue, justifying this situation, is that any waiver would have to refer to individual patents, which would be outside the realm of possibility as previously mentioned. But there are other, more significant and widespread causes. First of all, only the technologically most advanced regions, like North America, Western Europe, China, and Japan, are typically granted patents on so-called “enabling technologies” in the pharmaceutical industry.[53] Developing nations are normally not mentioned in such designations. Furthermore, knowledge plays a crucial part in the development of vaccinations; therefore, it is maybe useless to try to patent vaccines in nations where such knowledge cannot be assimilated. To this, it must be emphasized that many vaccinations, particularly mRNA vaccines, require cold chains and other organizational tools that are sometimes not feasible in developing nations. Significantly, according to a head of the UN-reporting organization known as the Medicines Patent Pool, not a single patent for mRNA vaccines has been submitted anywhere on the African continent.[54]
As was previously indicated, the patent situation is extremely problematic for vaccinations against SARS-CoV-2.
Both those using messenger RNA and those using an adenoviral vector, along with the others, are built on knowledge and technologies that have been gradually introduced over the years by a variety of different actors. The discovery of the virus in January 2020 allowed for the addition of the final contributions. Such a quick creation of these vaccinations would not have been conceivable without this pre-existing body of scientific knowledge.
This has the logical consequence that the vaccines themselves are, in one way or another, protected by patents or applications from numerous corporations, with claims that can occasionally be too broad to be avoided.
Regarding mRNA vaccines specifically, it should be noted that the combination of these with lipid nanoparticles (LNP) represents the development of studies that started more than forty years ago. It has been recognized to transport mRNA inside murine and human cells by causing protein synthesis since the late 1970s when it was encapsulated in liposomes.[55]
Several patents claiming LNPs or other lipid particles with specified properties mixed with mRNA or other types of RNA had been submitted in the later but still not recent years.
Some long-standing patent applications, which particularly address the use of mRNA as a vaccine, have gradually shifted their attention to the coronavirus family by means of continuations.
In particular, CureVac submitted an international application in 2007 that was published as WO2009030254 and whose main claim comprised a complexed RNA as its topic. Many years later, CureVac filed the US application US20210060175 through a series of continuations. In this application, CureVac carved out new claims specifically for SARS viruses while also retrieving certain information from the description of the original international application. Similarly, CureVac filed an international application in 2011 that was published as WO2012116811, in which it made extremely general claims about an mRNA vaccine. Years later, through a series of continuations, CureVac filed US application US20210032199, in which it carved out a new, more specific claim for vaccinations against SARS-CoV by using data from the description of the original application international application.
Moderna, on the other hand, is the owner of later patents, which since the initial application have a more focused scope. With regard to this, the main claim of the patent US10702600 (priority 2015) states: “Composition comprising a ribonucleic acid messenger (mRNA) comprising an open reading frame encoding for a S protein or for a subunit of beta-coronavirus S protein (BetaCov), formulated in a lipid nanoparticle“.
SARS-CoV-2 is a member of the betacoronavirus subfamily. Therefore, the other mRNA vaccines against SARS-CoV-2 can be included in this claim.
Additional issues with mRNA vaccines include its volatility and a few negative side effects. The inclusion of a modified uridine or pseudouridine was suggested in light of these long-known issues. The University of Pennsylvania (UPenn) has a number of patents pertaining to this, the most important of which is US 8278036 (priority 2005)[56], whose main claim relates to a “method for inducing a mammalian cell to produce a protein of interest, comprising contacting said mammalian cell with modified RNA modified RNA synthesised in vitro, encoding for a protein of interest, wherein said modified RNA comprises the pseudo”. Neither in this claim nor in others vaccination is expressly mentioned.
Both Pfizer-BioNTech and Moderna vaccines make use of 1-methylpseudouridine, which is also a modified uridine and hence falls under the above-mentioned patent.
Biontech has precisely secured a licence from UPenn[57]. For its part, Moderna, a few years after UPenn, had applied for a number of more specific patents, including US9428535 (priority 2011), claiming a “method for expressing a polypeptide of interest in a mammalian subject, comprising administering to said subject a isolated mRNA comprising … wherein said isolated mRNA is completely modified with 1-methylpseudouridine”.
Since the prior patent from UPenn explicitly mentions in the description that pseudouridine includes 1-methylpseudouridine, it appears that this patent is dependent on that one.
Further patents originate from research carried out in earlier years on the SARS Cov-1 and MERS (Middle East Respiratory Syndrome), by research groups from the National Institutes of Health (NIH) of the US Department of Health. In that research a mutation in the Spike protein was uncovered protein that compelled the protein itself to maintain a conformation that prohibited the virus from merging with the cell. It was then realised that, by blocking the Spike protein in that conformation, known as pre-fusion, one could allow more time for the immune system to produce antibodies against it. Proline was substituted for two amino acids at positions 968 and 969 in the aforementioned mutation (K968P and V969P substitutions). For this reason, we use the term “2P mutation.”
One result of this research was the application WO2018081318 (priority 2016) following which, in 2021, was issued patent US patent US10960070.
As a reminder, the sequencing of the SARS-CoV-2 virus was released in early 2020, therefore straddling between the filing and granted of this patent. Later, it was discovered that the identical 2P mutation (K968P and V969P) results in the same favorable features reported in the aforementioned application in reference to other coronaviruses even in the Spike protein of SARS-CoV-2. It was then discovered that the identical mutant protein is encoded by the mRNA in all three vaccines from Pfizer BioNTech, Moderna, and CureVac. And the DNA in Janssen’s adenoviral vector vaccine follows the same rules. Therefore, it is conceivable that all three businesses need Department of Health licenses.[58]
Following the deposition of the SARS-CoV-2 virus sequence, several companies filed patent applications within a short period of time: Moderna (WO2021154763, priority 28.1.2020), CureVac (WO2021156267, priority 4.2.2020), BioNTech (WO 2021213924, priority 22.4.2020).
In one way or another, each of these three applications makes a claim regarding the identical mutant 2P protein and/or the mRNA that codes for it. It appears that all three applicants combined the sequence of the 2P mutant protein with the information made available by the Department of Health[59] in the same way.
Evidently, the time period was too brief to collect experimental data; in fact, the topics covered in these inquiries are rather broad. At least the principal claims of all three inquiries were deemed to lack innovation or imaginative activity in international study reports. It remains to be seen if patents will be issued on the basis of them and with what claims, of course.
What is currently apparent is a fact that seems unusual. That is, despite the Covid vaccines’ immense value from a health perspective, it is by no means a given that they will enjoy substantial protection from a patent perspective.
Anyway, considering the evident importance and relevance of the recent global health crisis, in order to encourage investment and expand access to more inexpensive medications for the European population, the European Union is reforming the laws governing the pharmaceutical business. Covid and rare disease-related shortages of essential medications have brought to light a number of difficulties, including falling European production, serious challenges with supply chains, and a lack of readiness for international health emergencies. For a 136-billion-euro European market, the Commission appears to be preparing to publish a draft reform on April 26, 2023, which will include the most significant revision of the current medical regulations in 20 years. It is particularly important to draw attention to how, according to the Reuters news agency, one of the proposed changes would reduce the length of intellectual property (IP) protection for businesses that create and market medicines in Europe.
3.7. Pharmaceutical inventions and the complex relationship between industrial property rights and health protection
What for many is an “ideological” fight over the proposition that it is incorrect to recognize patent protection for such crucial assets at such a crucial time appears to ignore the significance of the tools made available for the protection of IP rights (in our case, the patent) in the context of the innovation undertaken by businesses.
Using the same terms used in the Manual of Industrial Law’s chapter on patents by A. Vanzetti-V. Di Cataldo, “Innovation is one of the essential moments of company activity. An entrepreneur who develops a novel concept and implements it into his company gains an advantage over rivals in the industry, which could make all the difference to his financial success.
“This is particularly important in the modern economy, which is characterized by a stable oversupply, and therefore by a situation of stable competition”, it is said again.
To put it another way, it becomes obvious that companies today operate in markets that are saturated in every product category, making it impossible to compete on price. Instead, it seems necessary to work in terms of research and innovation and, where possible, their implementation.
Innovation, particularly in today’s world, is not random. The competitive benefits that come from using an innovation are obviously crucial for the business that created it; nevertheless, it is clear that this advantage would be lost if everyone had access to the invention.
Again, it is hard to believe that some businesses would choose the route of innovation if protection in the form of patent rights were not granted in favor of the inventor, so losing the incentive for advancement and the resolution of new problems that need, by necessity, equally new responses.
Therefore, the purpose of patent protection, which is not simply to grant the inventor a legal monopoly, is to encourage innovation and progress. Two additional pillars of the protection provided by patents for inventions are that they encourage disclosure, which leads to the collective acquisition of the relevant discovery, and that they guarantee the validity of the invention.
It’s crucial to consider the industry in which patents are issued while discussing this type of protection.
It is actually not the same thing to discuss the protection given to inventions that, despite being disruptive, relate to broadly “technological” sectors and those that, from a very different perspective, belong to the pharmaceutical industry and, as a result, invoke extremely sensitive interests like health.
What is the reason behind this novel approach? There is little question that finding the perfect balance between vaccinations and patents requires a more delicate balancing act than it does in other areas of research.
This complexity arises from the value of the right to health and the potential restrictions it might face if, after filing for and receiving a patent, free movement and, consequently, accessibility to the pharmaceutical invention and its positive effects on health were hindered.
For many people, the universal right to treatment goes beyond simple ethical considerations to become an unavoidable requirement due to the complex predicament the pandemic has caused.
Now that we have established the theoretical validity of the arguments put forth by those who have argued that, in this context, it is necessary to derogate from the application of the industrial property protection regimes in order to renounce them, let us examine whether this validity continues with reference to some particular issues. To do this, we will highlight certain aspects that are typically absent from the opinions of those who support the “liberalization” of patents on anti-Covid vaccines.
The first thing to keep in mind is that releasing IP rights won’t increase the scale or speed of vaccine production and distribution.
The lack of raw materials, inadequate production capacity, and extremely complex manufacturing processes (in the case of mRNA and vector vaccines, that require a great level of sophistication and technology to produce, and they do so in bioreactors) are the main causes of the delays in vaccine production and dissemination, and these problems were already present in the early stages of the pandemic.[60][61] A waiver of IP protection is unlikely to be able to resolve these factual issues. Furthermore, it is critical to point out that efforts to scale up manufacturing shouldn’t prioritize quantity at the expense of pharmaceutical quality and safety.
The fact that contracts and collaborations are based on IP rights is a second factor to take into account. The new mRNA and vector vaccines have a very complicated product life cycle, from invention to post-marketing authorization safety assessments.[62] The level of collaboration during the design, manufacture, and dissemination of Covid-19 vaccines is unique. Partnerships for collaborative production (e.g., BioNTech/Pfizer/Sanofi/Novartis; CureVac/Bayer; Moderna/Lonza) and development (e.g., BioNTech/Pfizer; CureVac/GSK) are fundamental for the final product to be developed.[63][64]
Contractual transfers of the knowledge required to use a licensed technology are typically included with voluntary patent licences. During research and development (R&D), vaccine developers acquire a significant amount of knowledge required for vaccine production.[65] Such information is typically not included in patents, patent applications, associated scientific publications, or drug authority evaluation reports. When voluntary patent licenses are reached, non-disclosure agreements are used to convey knowledge. However, a patent waiver would eliminate any incentive for the creators of the original products to give such information to producers of biosimilars. The possibility that the waiver of trade secret protection might be successfully implemented and enforced to encourage businesses to divulge all pertinent know-how is extremely remote.
Another issue to take into account is that IP rights cannot be waived in place of regulatory requirements for vaccination authorization.
Any organization wishing to commercialize a medical product intended for human use, whether it be an original, generic, or biosimilar medicine, must first get marketing authorization from the relevant drug authorities. To prove that their biological product is “highly similar to the reference medicine” and that there are “no clinically meaningful differences between the biosimilar and the reference medicine in terms of safety, quality, and efficacy,” biosimilar developers must conduct extensive comparability studies with the “reference” biological product. [66] Not only in the EU or the US, but also in developing nations like South Africa and India, regulatory requirements for biosimilars must be followed.
Assuming the safety and quality criteria can be maintained, current vaccine developers can either transfer their marketing authorization license[67] or help their local partners get marketing authorization by providing the necessary information and know-how. However, due to the IP waiver, they might not be eager to comply in this area.
Therefore, each new applicant for a marketing authorization would need to adhere to safety, quality, and efficacy standards even if all associated intellectual property rights – including test data exclusivity – were waived. All of this shows that, if the present patent holders stop collaborating and/or delivering self-produced vaccines, a waiver would probably create a delay rather than hasten the availability of vaccines.
Furthermore, it is debatable whether a concession of IP rights will result in a considerable decrease in vaccine pricing.
It makes sense that people would be concerned about the cost of vaccines, given the disparities in access to healthcare between different nations. However, there are a number of reasons why a surrender of IP rights would not lead to biosimilar copies being significantly cheaper than the currently available drugs. First off, some modern vaccine inventors and producers have made public vows to operate “not for profit.”[68] Prices are likely to stay at a competitive level despite worries that such obligations may eventually be lifted because of the growing number of actual and hypothetical substitutes and the resulting competition.
In comparison to generic versions of small-molecule medications, the development and production of biosimilars are more expensive due to technological requirements.
Therefore, setting up the production for the new vector and mRNA vaccines calls for large investments. Like the original corporations, biosimilar and generic businesses typically pursue profits. Although the market costs for these goods may not be considerably less than the prices of vaccines today, the waiver would primarily serve the business interests of the generic producers as they would not be required to pay royalties.
It seems doubtful that such pricing would be significantly less than the existing rates for vaccines delivered under the not-for-profit obligations, even if generic producers were willing to limit prices to their own production costs. A waiver might serve the public interest in inexpensive vaccinations more than it would the economic interests of biosimilars makers unless they agree to sell at cost.
Third, the price of vaccine distribution alone — excluding the price of manufacture — is high.[69] It may occasionally be equal to 50% of the market price of the vaccination. Regardless of whether vaccinations are covered by intellectual property protection, every entity along the intricate supply chain needs to be compensated for the goods and services they provide.
When the vaccinations were still in the research and development stage, there was certainly a risk of inflated prices. Governments should have addressed this issue as part of the agreements supporting the funding of vaccine research and the cost of vaccines should be tackled as a question of global solidarity.
Finally, the incentives for developing new drugs will probably suffer from a widespread waiver of IP rights. A comprehensive IP waiver’s possible implication on the incentives for innovation in vaccine development and other fields of medical research must be taken into account. The first Covid-19 vaccinations were licensed years before the outbreak, thanks to innovations like Moderna’s mRNA-1273 technology[70] and BioNTech and Pfizer’s BNT162[71], which should be emphasized. This suggests that the research that led to those discoveries was not focused on the vaccines that are currently being used to combat the epidemic. These platform technologies could lead to a wide range of therapeutic uses in various fields of medicine, such as the treatment of cancer.[72] A waiver of IP protection would not be in the best interests of society because it would discourage businesses from investing in such areas of research.
The accountability for the use of public money invested in the development of vaccines, as well as the linked and desired transparency, should be our final point of discussion.
Both commercial and public funds are frequently used to fund the research of new drugs. While public organizations, like as universities and research institutes, are normally responsible for funding and carrying out basic research, the private sector, specifically pharmaceutical corporations, are typically responsible for funding and carrying out late phases of the development process. Accountability is a problem since public funds are used in drug development. This calls for openness on the financial commitments made and the contract reached for the exploitation and sale of a drug or vaccine created with public funds.
The question of what constitutes the ideal balance between the free access to vaccines (with a context-specific waiver of IP protection) and the protection of industrial and intellectual property rights thus seems to be a challenging one to address precisely.
3.8. Competition in the regulatory and economic landscape of Covid-19 vaccines
A large-scale public health crisis-related vaccine race will experience attrition, which is an expected and, in some cases, unavoidable phenomena. The sheer number of participants in the COVID-19 vaccination race, as well as how swiftly discrete multi-party R&D collaborations came together, set it apart from earlier races.[73]
Intense negotiations have been conducted by the European Commission to create a diverse portfolio of vaccines for EU residents at reasonable costs. Contracts with eight talented vaccine developers have been reached, securing a portfolio of up to 4.2 billion doses.
Eight conditional marketing authorizations for vaccines created by BioNTech and Pfizer, Moderna, AstraZeneca, Janssen Pharmaceutica NV, Novavax, ValnevaSanofi-GSK, and HIPRA have thus far been approved by the Commission following favorable safety and efficacy evaluations from the European Medicines Agency (EMA). in addition, the EMA is evaluating other vaccinations at various stages.[74]
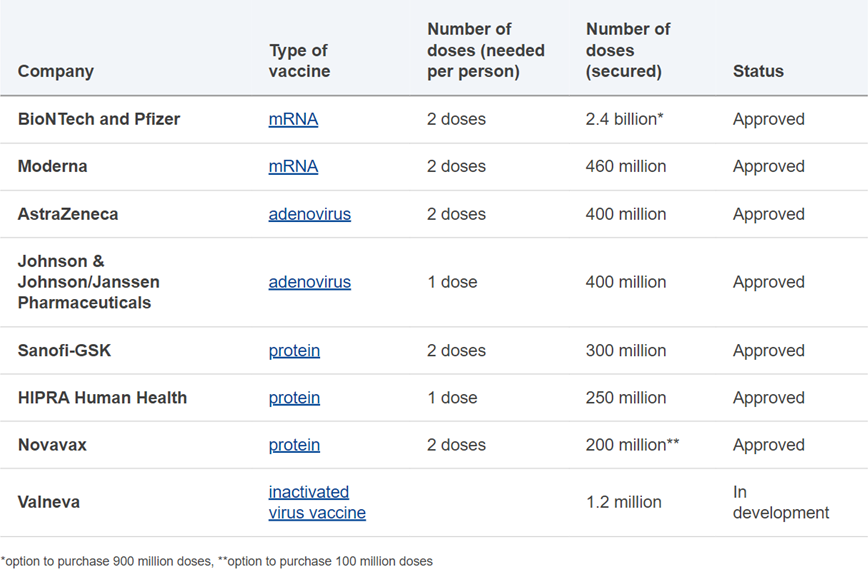
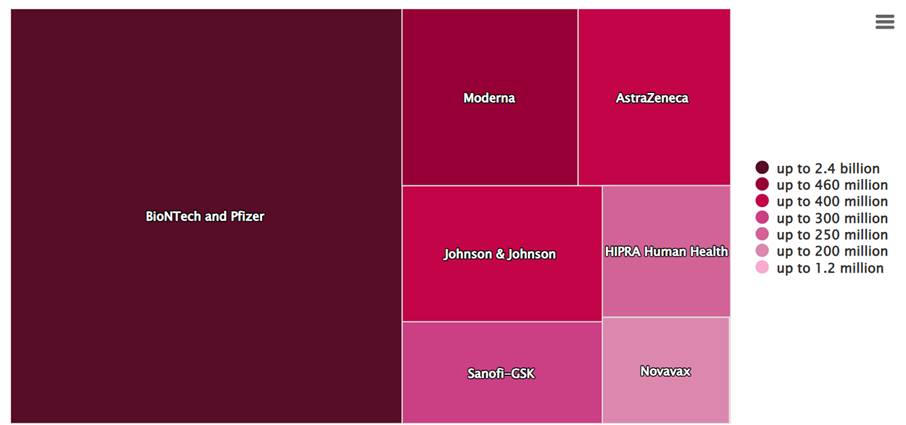
Figures: European Union vaccine portfolio and doses[75]
Let’s now examine each of the major producers individually, paying particular attention to the businesses which manufactured mRNA vaccines.
Pfizer/ BioNTech
Early in 2020, the biotech company BioNTech, founded by Özlem Türeci and Uğur Şahin, developed a COVID-19 messenger ribonucleic acid (mRNA) vaccine. On March 17, it made public a partnership with Pfizer, under which the big pharma firm would help with manufacturing and clinical research for all countries outside of China. The two businesses had previously conducted business together; for instance, in August 2018, they signed a collaboration agreement to create mRNA-based vaccinations for the prevention of influenza.
With approval from the MHRA, the FDA, the EMA, and the WHO in December 2020, the Pfizer/BioNTech candidate, known as Comirnaty, was the first vaccination to be authorized for use in an emergency situation by four of the major agencies.
Pfizer and BioNTech had started establishing their supply chains for vaccines far earlier. Initial production would occur through a network of already-existing facilities, the majority of which are owned by Pfizer. At a facility in Missouri, Pfizer initially created the initial stage of the drug product (DNA plasmids). Then, these plasmids were delivered to two facilities—a Pfizer facility in Andover, Massachusetts, and a BioNTech facility in Mainz—frozen, packaged, and shipped. The mRNA, the active medicinal ingredient, was created at such plants by converting the DNA. Bags of filtered mRNA were then delivered to two more locations for the formulation, fill, and finish stages. The Mainz mRNA was sent to a Pfizer factory in Puurs, Belgium, and the Andover mRNA was sent to a Pfizer site in Michigan. The vaccine vials were then packaged and distributed abroad after that.[76]
Large quantities of lipid nanoparticles were necessary for the formulation created at the facilities in Michigan and Belgium in order to mix with the mRNA. The supply chains for lipids were unique and specialized. Acuitas, a Canadian company, provided BioNTech with technology under a license, but the lipids were ultimately produced at scale somewhere else. A five-year contract was inked in November for Avanti Polar Lipids of Alabama, a Croda subsidiary in the UK, to produce Pfizer’s lipids. Following that, BioNTech entered into agreements with companies like Evonik and Merck KGaA to produce lipids at locations inside the European Union.
Demand surged as a result of the Pfizer/BioNTech vaccine’s early results. To improve capacity, the corporations widened each section of their US and European supply chains. In addition to adding more vaccine formulation capacity in Michigan and extra fill and finish at a different site in Kansas, Pfizer stated that it would start producing lipid nanoparticles at one of its facilities in Connecticut. In order to scale up manufacturing, it also hired Exelead, a CDMO with background in lipid nanoparticle production. Pfizer started using one of its facilities in Ireland for Europe, and BioNTech’s recently acquired Novartis facility in Germany started operating in February 2021. Thermo Fisher, Siegfried, Delpharm, Sanofi, Novartis, and Thermo Tech were all contracted by BioNTech to fill and finish in various plants throughout Europe, relieving some of the pressure off the Pfizer facility in Belgium, which nevertheless also increased capacity.
The BNT162b2 (Comirnaty) mRNA vaccine is based on messenger RNA (mRNA) technology, which inoculates the genetic sequence with the instructions to make the antigen rather than the antigen toward which an immune response is to be produced. The generated antigen is then expressed in the vaccinated person’s cells. The SARS-CoV-2 spike protein, which is present on the virus’s outer surface and is used to enter cells and reproduce, is encoded by messenger RNA found in the Comirnaty vaccine.[77]
The majority of the information included in the EMA’s evaluation came from a global clinical study that was published in the New England Journal of Medicine on December 10, 2020. The study’s main goal was to evaluate the BNT162b2 vaccine’s safety and effectiveness against symptomatic COVID-19 disease that had been confirmed in a lab setting.
The research revealed that during the time that the original WUHAN virus strain was in circulation, 95% of laboratory-confirmed symptomatic COVID-19 cases in people 16 years of age and older might have been avoided with the Comirnaty (BNT162b2) vaccine.
The spread of new viral strains has, however, altered the statistics on vaccine efficacy. The epidemiological surveillance bulletin contains weekly estimates of the vaccine field efficacy data, or “in real life.”
Moderna
Cambridge, Massachusetts-based biotech startup Moderna was established in 2010. Moderna created a potential mRNA vaccine in association with NIH researchers. It initially partnered with PPD, a contract research organization, to support its Phase 2 and 3 trials. On December 18, 2020, the FDA approved Moderna for emergency use.[78]
In comparison to Pfizer and BioNTech, Moderna created its manufacturing supply chain using a very different strategy. It had to start from the beginning, in contrast to those businesses. Moderna had a plant in Massachusetts where it produced smaller batches of their vaccine for clinical studies, but that facility was insufficient for manufacture at a commercial scale. It partnered with Lonza, a large CDMO, and they signed a 10-year strategic agreement on May 1, 2020.
For vaccine sales intended for markets outside of the US, Lonza constructed production lines at a factory in New Hampshire that received some assistance from the US government as well as at another site in Switzerland. Moderna worked with CordenPharma, another CDMO, to produce the massive quantities of lipid nanoparticles needed for the vaccine’s mRNA nature. CordenPharma, which might produce from facilities in Colorado, Switzerland, and France, and Moderna previously worked together. For the initial fill and finish of Moderna’s vaccine, Catalent in the US and Rovi in Spain were responsible for the European supply chain.[79]
Following its initial success, Moderna sought to grow as the demand for its vaccine rose. Moderna collaborated on a drug substance in Europe with Rovi, in one of its sites in Spain, and Lonza, at a different factory in the Netherlands. In order to enhance its capability for local manufacturing, Moderna declared it will remodel its Massachusetts facility. For the US supply chain and the European supply chain, fill and finish would be extended to facilities controlled by Baxter, Sanofi, and Thermo Fisher and Recipharm in France.
The anti-COVID-19 mRNA-1273 vaccine Spikevax (formerly known as the Moderna vaccine), has been recommended for release in constrained commercial settings as of February 6, 2021 by the European Medicines Agency (EMA).
The Spikevax vaccine is based on messenger RNA technology, much as the Comirnaty created by Pfizer/BioNTech; the mRNA encodes for the spike protein of the SARS-CoV-2 virus. Therefore, the vaccine only delivers the genetic material needed by the cell to produce copies of the spike protein; it does not actually introduce the virus into the cells. Shortly after immunization, the utilized mRNA is broken down and leaves the body.
The vaccine showed 94.1% efficacy in preventing symptomatic SARS-CoV-2 infection compared to placebo, in line with the efficacy of Pfizer/BioNTech’s Comirnaty, the first vaccine approved in the European Union and based on the same mRNA technology. Overall, 11 cases of COVID-19 with onset at least 14 days after the second dose were recorded in the group that received the vaccine, compared to 185 in the control group. Participant stratification based on age (more than/equal to or less than 65 years), co-morbidities (chronic lung illness, heart disease, severe obesity, diabetes, liver disease, HIV positivity), gender, and ethnicity also confirmed the same efficacy.[80]
AstraZeneca/Oxford University
When Oxford University researchers publicly announced the identification of a vaccine candidate in March 2020, the AstraZeneca vaccine tale officially began. The professors used their personal contacts to get in touch with Merck, a multinational pharmaceutical business with its headquarters in the United States, as they lacked experience with large-scale distribution. Due to a number of factors, including the British government’s reservations about securing the vaccine entirely from a US corporation in light of the Trump administration’s America First agenda, those negotiations are said to have fallen through.
Oxford joined forces with AstraZeneca on April 30, a British-Swedish pharmaceutical business with global operations and a Cambridge, England, headquarters. Oxford Biomedica agreed to make the vaccine for clinical trials in May, and Symbiosis Pharmaceutical’s Scottish facility agreed to complete the fill-and-finish work in June. Cobra Biologics, UK, agreed to create the therapeutic product on a commercial scale in England, and CP Pharmaceuticals was hired to complete the fill and finish in Wales.
Despite growing pains with its US, Indian, and European supply chains and public health concerns, AstraZeneca’s vaccine continued to play a significant role in the fight against the pandemic on a global scale. The business entered into agreements with multiple additional partners to expand its supply chain elsewhere.
The European Medicines Agency (EMA) recommended on January 29, 2021 that the ChAdOx1-S vaccine, created by Oxford University and AstraZeneca, be given a conditional marketing authorization for the prevention of COVID-19 in people ages 18 and older. This recommendation followed a thorough evaluation of quality, safety, and efficacy data. The EMA authorized the marketing name “Vaxzevria” for the vaccine, also known as AstraZeneca’s COVID-19 Vaccine, on March 26, 2021. It was the third COVID-19 vaccine, after those made by Pfizer/BioNTech (approved on December 21, 2020) and Moderna (Spikevax, approved on January 6, 2021), to be licensed for marketing by the European Commission following a favorable EMA judgement.
Vaxzevria takes a distinct approach from the mRNA-based Pfizer/BioNTech and Moderna vaccines in order to stimulate the body’s immune response to the spike protein. A modified chimpanzee adenovirus that is unable to replicate is used as the viral vector vaccine’s vector to deliver the instructions for synthesising the SARS-CoV-2 spike protein. Once generated, the protein can prompt a particular immunological response, including cellular and antibody responses. By the end of 2019, the first Ebola vaccine was authorized using the same technology. The adenoviral vector was created in cooperation with Advent-IRBM of Pomezia. With this technology, the vaccine is more stable than mRNA vaccines and doesn’t need to be stored or transported at extremely low temperatures. Vaxzevria’s benefit/risk balance is confirmed to be favourable because it significantly lowers the risk of developing a serious illness, needing hospitalization, and passing away from COVID-19. As a person gets older, this balance seems to get better. The use of the same technology against other viruses, such as HIV and Zika, is also being studied. [81]
Johnson & Johnson/Janssen
In what was subsequently known as Operation Warp Speed, Johnson & Johnson was the first vaccine manufacturer to get funding from the US government in the months of February and March 2020. The vaccine was created in cooperation with Beth Israel Deaconess Medical Center of Boston by Janssen Pharmaceutica, a branch of Johnson & Johnson with headquarters in Belgium, and the candidate was unveiled on March 30. A first batch of clinical trial supplies was produced in a Johnson & Johnson facility in the Netherlands. With news of collaboration with Emergent BioSolutions to produce drug substance and Catalent to perform fill and finish in Indiana, the supply chain in the United States started to take shape in April.
The Catalent agreement was expanded in July to include its Italian facility; in September, fill and finish services were also contracted with Grand River Aseptic Manufacturing (GRAM) in Michigan. Production of the medicinal material began in August after the US government agreed to buy 100 million doses. Johnson & Johnson and Merck signed a contract in March 2021, with US government assistance, for the manufacture of the drug material at a Merck factory in North Carolina as well as fill and finish at a Pennsylvania facility. The supply chain across Europe was organized to receive the drug ingredient from the Leiden factory. Johnson & Johnson also secured agreements with Reig Jofre in Spain, Sanofi Pasteur in France, and IDT Biologika in Germany for fill and finish in December 2020, February 2021, and March 2021, respectively. The Johnson & Johnson vaccination encountered difficulties even though the US and European supply chains appeared to be set up successfully.
The Janssen vaccine is a viral vector made of a recombinant human adenovirus type 26 that is incompetent for replication and has been appropriately modified to contain the gene encoding for the entire sequence of the spike protein (S) of the SARS-CoV-2 virus in a stabilized conformation (vaccine Ad26.COV2.S). The coronavirus enters human cells by means of the spike protein found on its outer surface. It only requires one dose, unlike earlier immunizations. In people over 18 (including subjects under 60 years of age), the study demonstrated an efficacy of 66.9% (95% CI 59.0-73.4) in avoiding the development of moderate to severe/critical COVID-19 disease 14 days after immunization, and 66.1% (55.0-74.8%) 28 days following vaccination. The most severe forms were successfully prevented, and effectiveness rose to 76.7% 14 days and 85.4% 28 days after administration. According to age, the existence of comorbidities, gender, and ethnicity, vaccine efficacy was comparable across all tested groups.[82]
- Economic analysis of Covid-19 vaccines
The pharmaceutical industry is one of the most profitable business sectors in the world, arguably more lucrative than the energy and banking industries.[83]
The expansion of pharmaceutical companies’ cash reserves and the rise in dividend payouts to shareholders are clear indicators of their growing wealth. Pharmaceutical firms are also progressively spending billions of dollars to acquire rival businesses in order to stifle competition, expand their patent portfolio, and develop new medication pipelines.[84]
Governments invested an unprecedented amount of tax dollars on the COVID-19 pandemic to fund the creation of vaccines through grants and Advanced Purchase Agreements (APAs) with private corporations.
Based on the vaccines market study published on January 2023[85], by Future Market Insights (ESOMAR certified market research organization, member of Greater New York Chamber of Commerce), the vaccines market is expected to expand its borders at an average CAGR of 6.6%. The market is estimated to reach a value of US$ 80.8 billion by 2033 after being valued at US$ 42.7 billion in 2023.
According to the market research study on vaccinations, the growth of the Covid-19 virus into a full-fledged pandemic has increased demand for vaccines. Government spending on innovative healthcare systems is also expected to increase the size of the vaccine market. The global increase in disease prevalence is promoting vaccination use, opening up opportunities for the vaccines business to expand. Due to the high mortality rates of deadly diseases like tuberculosis, cancer, influenza, and pneumonia, many people choose to get vaccinated. The market for vaccines is also anticipated to rise as a result of increasing government investment in research and development as well as rising public awareness of the advantages of immunization.
Following the mentioned market research, from 2023 to 2033, the vaccines market is anticipated to rise as a result of a number of important factors, including the increased prevalence of chronic and infectious illnesses, an increase in immunization programs, technological advancements in the field of vaccines, and shifting public opinion regarding vaccines. Giants in the vaccination industry like Pfizer and GSK are actively investing in vaccine research and development in order to launch new medications that are incredibly successful. Additionally, they are conducting numerous trials to determine the safety and effectiveness of vaccines.
Trends in the vaccination business are significantly impacted by rapidly changing technologies. Lab automation and the introduction of genetic engineering are enabling vaccine producers to create less expensive vaccinations, which may draw customers and eventually open up the vaccine market.
High capital investments and low purchasing power in emerging markets, on the other hand, may impede market development and pose a challenge to the growth of the vaccine market. The process of creating vaccinations is time-consuming, challenging, and expensive. As a result, the majority of vaccinations are often more expensive, which is posing a significant barrier to the expansion of the vaccines market. Similarly, poor healthcare facilities and a lack of understanding have restricted access to the vaccine market throughout emerging nations.
The vaccine market is quite complex. Since vaccinations are mostly used prophylactically, or to prevent diseases, the pharmaceuticals market gives the vaccines segment a unique position. The pharmaceuticals described in most of the other segments are only administered if you already have a condition, thus only specific categories of people are possible patients. This also implies that they are administered to a bigger portion of the population. The market for vaccines has very few significant competitors.
Due to COVID-19 vaccinations, a few new players have entered the market, and this has significantly accelerated growth. Despite the intense time constraints, they were developed, and billions of doses have been generated.
In the upcoming years, demand for COVID-19 vaccinations is expected to remain quite high. The mRNA-based COVID-19 vaccines are the first medicines of their sort to be authorized, advancing this field of study. Numerous diseases, including cancer, psoriasis, malaria, HIV, osteoarthritis, and Alzheimer’s disease, as well as vaccines against other infectious diseases, have very bright futures.
- Covid-19 related profits
The multinational pharmaceutical companies Pfizer, BioNTech, Moderna and Sinovac have made USD 90 billion from Covid-19 vaccinations and medications in 2021 and 2022. In its report ‘Pharma’s pandemic profits’ published on 27 February 2023, Somo, a Dutch organisation that investigates the behaviour and policies of large transnational corporations, highlighted how companies have achieved these exceptional gains in great part because of “decades of research funded by public investment, billions in grants for development and production, and tens of billions in Advanced Purchase Agreements (APAs)”.
In more detail, Pfizer generated net profits of $25 billion, BioNTech and Moderna $20 billion apiece, while China’s Sinovac posted margins of around $15 billion. Researchers from Somo examined the financial records of the top seven producers of Covid-19 vaccines, a business that made $86.5 billion in revenue and $50 billion in profit in 2021 alone. However, just four businesses—Pfizer, BioNTech, Moderna, and Sinovac—realized sizable profits. The pharmaceutical industry, one of the most lucrative economic sectors in the world, saw profits that were even higher than the already high profits of usual business, according to the report. The margins for 2021, however, are even between 62% and 76% if only Pfizer, BionTech, Moderna, and Sinovac are taken into account. Equally high are the profits for the four firms that have made the greatest amount of revenue from the sale of Covid-19 vaccinations in the first nine months of 2022, which were estimated by Somo to be USD 30 billion. These are particularly startling numbers when we realize that only Pfizer had generated revenues prior to even beginning the development of Covid-19 vaccines. AstraZeneca and Johnson & Johnson, in contrast, stated that they would distribute their vaccines on a non-profit basis. The analysis for this study reveals that these businesses have, at most, produced modest profits. Sales of Novavax’s vaccination products just began in 2022, and thus yet, the company has not generated large revenues.[86]
In 2021 and 2022, the net profit margins from COVID-19 vaccines and treatments were expected to range from 49% to 76%, which is significantly greater than the industry average margin for the pharmaceutical industry. However, compared to Q1–Q3 of 2021, the net profit margins for Pfizer/BioNTech and Moderna did decline.
With a market share of 67% for Pfizer/BioNTech and 26% for Moderna as of 12 October 2022, the data that are currently available demonstrate the dominance of Pfizer/BioNTech and Moderna’s vaccines and boosters inside the USA and European Union.
69% of Moderna’s product sales in 2021 came from the European Union (31%), the USA (30%), and the rest of Europe (8%). 58% of Moderna’s product sales during the first nine months of 2022 came from the European Union (33%) and the US government (25%).[87][88] In contrast to the first nine months of 2021, 47% of Pfizer’s Comirnaty revenues were reportedly produced in the USA and in “Developed Europe” during the first nine months of 2022.[89][90]
- Covid-19 vaccine companies and the subsidies from governments
For the development of COVID-19 vaccines during the pandemic, numerous firms received sizable funding. Most of the money went toward improving production capabilities and funding clinical investigations. Government financing for the development of COVID-19 vaccines and medications totalling at least USD 5.8 billion was given to the seven main private COVID-19 vaccine manufacturers.[91]
The seven major private COVID-19 vaccine makers were each given a USD 5 billion funding from the US government, which was by far the largest funder. Under Operation Warp Speed (OWS), a US government initiative that was started in May 2020, the money was made available. Six vaccines with the highest priority were chosen from OWS. AstraZeneca, Johnson & Johnson, Pfizer/BioNTech, and Moderna were chosen among three different vaccination technologies: protein (Novavax, Sanofi), viral vector (AstraZeneca), and messenger RNA or mRNA (Pfizer/BioNTech and Moderna).
Pfizer did not accept donations from OWS or any other donors. However, it did gain from tens of billions in advanced financing provided by APAs.
In order to develop its mRNA vaccine, Pfizer’s rival Moderna accepted USD 1.7 billion in funding from the US government in the years 2020, 2021, and 2022. In addition, Moderna benefited from significant upfront funding provided by APAs. These companies’ 2021 revenue records show that APAs contributed significantly to that total.
Pfizer nonetheless benefited indirectly from the USD 0.4 billion supplied by the German government as a result of its work with BioNTech on the Pfizer/BioNTech vaccine, despite having rejected US government funding for vaccine development.[92][93]
With BARDA in the USA, Moderna entered into a number of grant agreements. The agreements between 2020 and 2022 cost about USD 1.7 billion in total. Moderna stated that it still has USD 67 million of the BARDA funds available as of September 30, 2022.[94][95]
To create NVX-COV2373 and distribute it through the COVAX facility, Novavax got up to USD 0.4 billion from the Coalition for Epidemic Preparedness Innovations (CEPI) in addition to the USD 1.3 billion of OWS.[96][97] According to Novavax, as of September 30, 2022, OWS funding of USD 0.5 billion (including grants and APAs) was still available.[98]
Speaking of AstraZeneca, it reported receiving USD 1.043 billion in government grants for the creation of vaccines and medicines in its annual reports for 2020 and 2021.[99] Sadly, AstraZeneca’s quarterly reports for 2022 do not specify how many additional government funds were recorded as revenue in 2022. Additionally, AstraZeneca does not make it known whose governments have given money. Since all agreements were made through BARDA, the US government is probably the only one involved.[100]
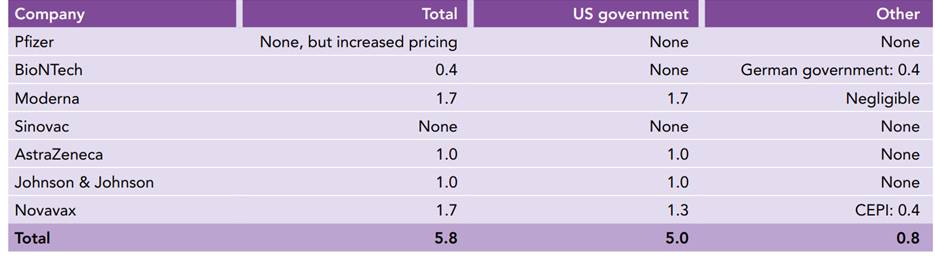
Table 1: Government grants to the seven vaccine producers for developing COVID-19 vaccines and medicines in USD billion[101]
Beyond the aforementioned seven businesses, government funding was used to create COVID-19 vaccines. This significantly hastened the creation of numerous vaccinations. Around 58 vaccines from different vaccine developers had been authorized for usage as of the time of writing.[102] Among them were the drug manufacturers Curevac, Merck, Sanofi, and Innovio.
Now let’s think about the APAs. APAs are contracts to purchase vaccinations before they have received approval, reducing the risk of vaccine development and production expenses. Most APA contracts are not accessible to the general public.
Compared to government grants, APAs were a considerably more significant source of funding. Different governments, the European Union, and COVAX entered into APA contracts that provided up-front money for vaccine development. Tens of billions of dollars were sent through APAs to pharmaceutical firms while the vaccines were still being developed.
There is no official record of all APAs, hence estimates of the exact amount paid range. A study conducted in January 2021 by the kENUP foundation estimated a total public expenditure of USD 93 billion for the development of vaccines and medications, of which USD 86.5 billion was funded by APAs.[103] Then, the Global Health Center’s estimated USD 45 billion[104], but it was based on only 26 APAs, which is a much smaller number than the total number of APAs that were ultimately concluded.
It was difficult to monitor whether public monies were used wisely and equitably, the cost of the vaccines, and whether agreements on equitable distribution had been signed due to the opaque nature of APAs and their contents. Many nations found it challenging to understand price agreements and negotiate a fair price due to a lack of transparency.
- Covid-19 vaccine companies and the profit spending
Pfizer, BioNTech, Moderna, and Sinovac are the four firms with the highest profits; they are also listed on stock exchanges. Their shareholders have received a sizable chunk of those gains.
More than half of Pfizer’s overall profits—approximately USD 35 billion for the years 2021–2022—came from COVID-19 profits. The additional revenue from COVID-19 vaccines and medications has not resulted in a significant increase in dividends. Pfizer distributed between USD 8 billion and USD 9 billion in dividends to its common stockholders throughout the course of the three fiscal years 2019, 2020, and 2021. The payment in 2022 was $9 billion USD. However, Pfizer did repurchase shares.[105]
A special cash dividend and a share repurchase were both announced by BioNTech in March 2022. For the period ending October 10, 2022, they repurchased shares for a total of USD 1 billion and paid a dividend of USD 486 million. Additional USD 0.5 billion in repurchases occurred between 7 December 2022 and 17 March 2023.[106]
Moderna did share repurchases but did not pay cash dividends. It stated in February 2022 and August 2022 that it would buy back shares for USD 1 billion, USD 3 billion, and USD 3 billion, bringing the total to USD 7 billion for an expected 1.5 years.[107]
A total of USD 14.7 billion in net profit for Sinovac in 2021 was made up of USD 6.1 billion in non-controlling interests held by Sinovac and its subsidiaries. Sinovac did not disclose any dividend payments or share repurchases, yet a profit of USD 8.6 billion remained attributable to its shareholders.[108]
Another aspect that is fundamental for these companies is R&D, regarded as the vitality of the pharmaceutical industry.
For Pfizer, the cost of R&D increased by USD 4.4 billion in 2021 compared to 2020.[109] They then reduced their R&D spending in 2022 compared to 2021.[110] The cost to develop the COVID-19 drug Paxlovid increased as a result of an acquisition, payments for license and collaborative agreements with other businesses, and additional spending. Costs associated with Pfizer’s COVID-19 vaccine collaboration deal with BioNTech were one of the primary causes of its expenses rising by USD 1 billion in 2020 compared to 2019.[111][112]
Moderna and BioNTech are gradually boosting their R&D expenditures. However, given that these businesses are expanding their medication pipelines, it is unclear how much R&D spending is tied to COVID-19 vaccines and how much additional R&D is generated as a result of the revenues realized in 2021 and 2022.
US government financing for the COVID-19 vaccine totals about USD 1.5 billion in Moderna numbers for 2020 and 2021 combined, but it is much less in 2022. However, some COVID-19 vaccination spending continued in 2022.[113]
In addition, Moderna assigned three non-COVID-19 vaccines to phase 3, the final stage of clinical testing, between October 2021 and November 2022. The mRNA technology is also the foundation for all three vaccines.[114]
The German Federal Ministry of Education and Research has provided USD 0.4 billion in financing to BioNTech to support its COVID-19 vaccination initiative during the course of 2020 and 2021 combined, but the amount of the increased R&D spending for COVID-19 vaccines is not disclosed by BioNTech in its quarterly report for the period ending 30 September 2022.[115] One non-COVID-19 vaccine is now undergoing phase 3 clinical studies. The seasonal influenza vaccine is likewise based on mRNA technology.[116]
An additional operation usually realised by pharmaceutical industries is M&A. Large pharmaceutical companies are increasingly investing less in internal R&D activities and more in acquiring smaller businesses to restock their pipelines.
For instance, in 2022, Pfizer successfully acquired Biohaven (a maker of migraine medications), Arena Pharmaceuticals, and Global Blood Therapeutics for a total of $12.8 billion, $6.4 billion, and $5.6 billion, respectively. [117]
Pharmaceutical corporations search outside of their own R&D divisions for novel medications. For instance, they can employ partnership agreements to gain access to inventions. Typically, this occurs prior to the clinical stages. More M&A transactions and larger pharmaceutical corporations acquiring smaller businesses to further drug development occur when there is clinical evidence.
Finally, the profits and/or stock value of the company were also profited by the management.
Albert Bourla, the CEO of Pfizer, obtained tens of millions of dollars and, in addition to his target award of 250%, which was worth USD 8 million, he received his salary of USD 1.7 million.[118] The same situation occurred in BioNTech and Moderna. Both their CEOs, Prof. Ugur S. and Mr. Stéphane Bancel, became billionaires, with assets totalling a combined 6.1 billion and USD 5.8.[119]
- Biopharma’s stock market winners in 2022, the flotations fail and venture investments
Stock market
Big Pharma lost $199Bn in combined market cap in the first 3 months of 2023, following Evaluate Pharma’s First-quarter round up infographic.
To finish the previous year on the stock market during a difficult period for biopharma, there had to be clear pipeline advancement. This challenge was overcome by companies like Daiichi Sankyo, Orion, Verona, and Madrigal who all saw significant share price increases in 2022.
On the other hand, the epidemic players had a sharp decline after reaching dizzying heights in 2021. These developers, which also include Biontech, Moderna, and Novavax, predominate the list of stock market losers, with a few additional names falling victim to good old’ clinical failures.
Merck & Co. rocketed to the top position amid a widespread end-of-year boost for large caps. Drivers are clearer for companies like Lilly, Abbvie, and Daiichi as they are all developing medications with high expectations.
Sarepta, one of the mid-caps, had its stock rise as a result of significant advancements in its gene therapy for Duchenne muscular dystrophy. Meanwhile, Halozyme is essential to the continued success of numerous popular cancer antibodies. A contender for treating mid-stage prostate cancer that Merck licensed after paying a hefty sum is giving Finland’s Orion a boost. Additionally, among the tiny caps, Imcrivee’s US and European approvals for the rare genetic disorder Bardet-Biedl syndrome helped Verona rise on COPD data, Madrigal score a rare victory in Nash, and Rhythm rise over the course of the year.[120]
Table 1: Biopharma’s biggest stock market winners of 2022[121]
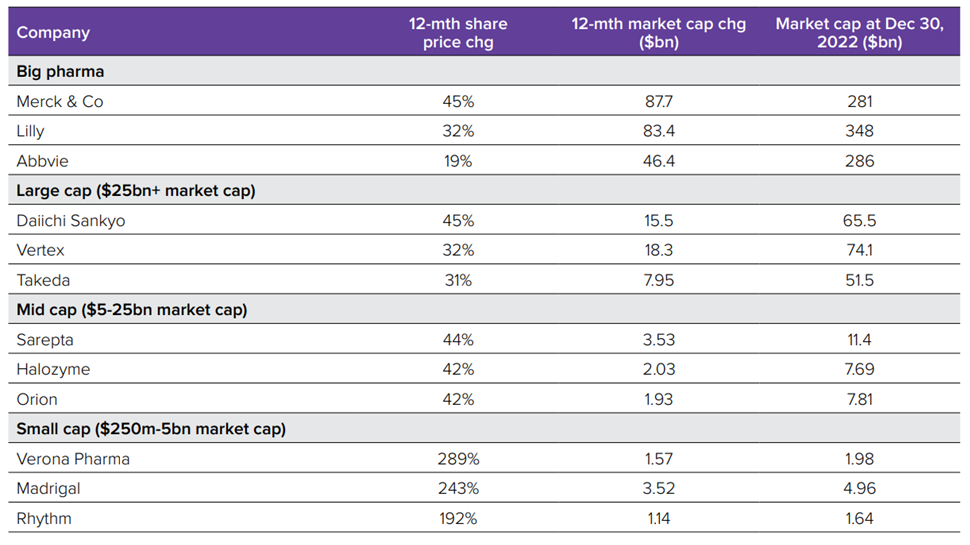
Table 2: Biopharma’s biggest stock market losers in 2022[122]
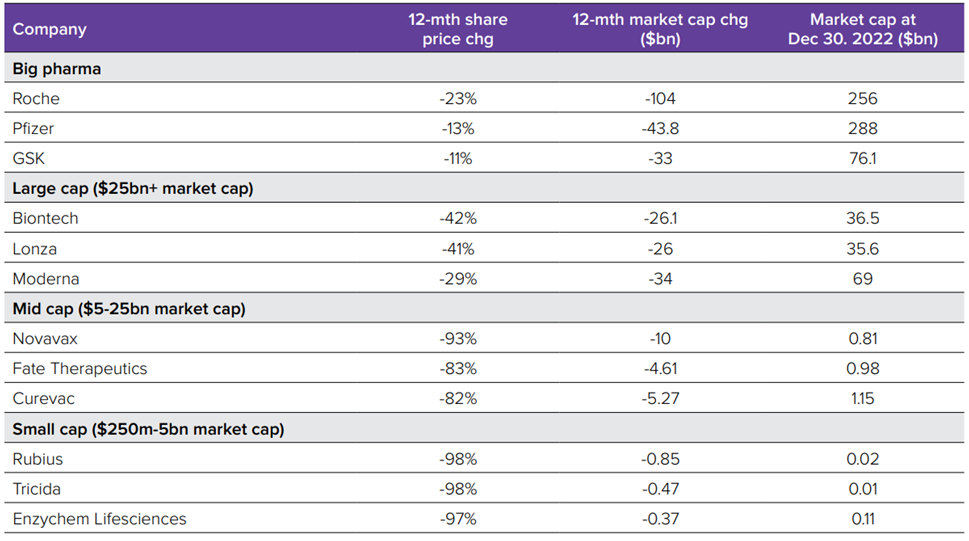
Flotations
The likelihood of biopharma going public was all but erased by a disastrous year for the industry’s stock markets. Only three groups were able to escape in the fourth quarter, as seen in table 4. Since 16 groups went public in 2012, last year was the worst for pharma developer flotations since that year. Furthermore, Third Harmonic, one of the IPO success stories from the previous year, is now in crisis, dealing still further blow to the industry.[123]
Table 3: Biggest biopharma IPOs of 2022[124]
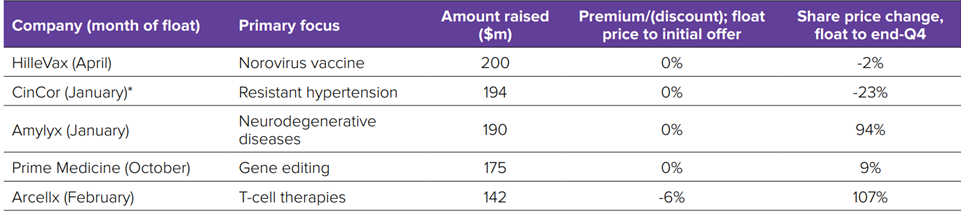
Table 4: Biotech IPOs by quarter on Western exchanges[125]
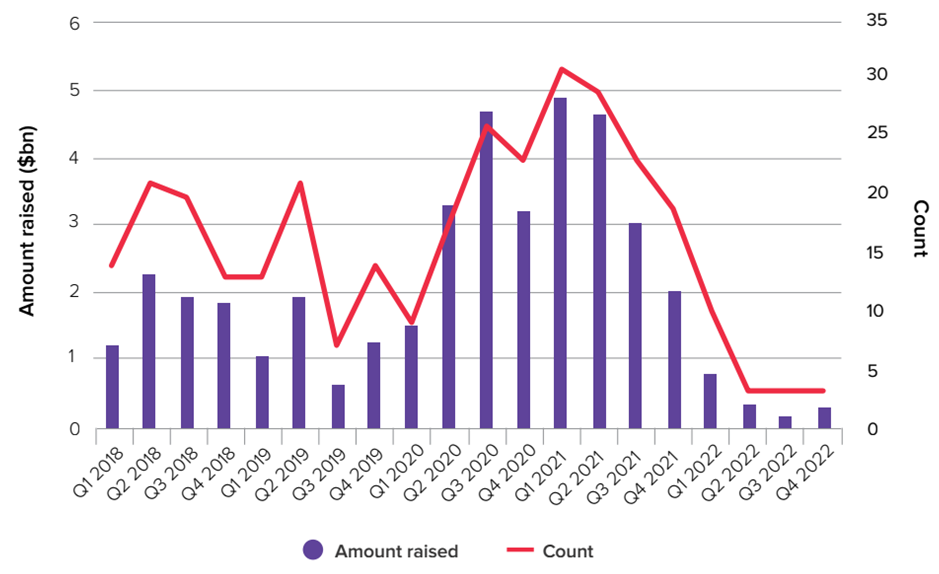
Venture capital investment
Investment in venture capital has returned to 2019 levels. This much is clear from the most recent data available, which shows the final three months of 2022 as the weakest quarter for drug developers in three years.[126]
Venture funds are currently fully filled, as demanded by investors. But as the IPO window closes and risk appetite shifts more broadly, businesses are being forced to gather their resources and defend their current portfolio companies. Investments are unlikely to rise until these circumstances shift.
How much lower may these statistics go is the crucial question. Although there has recently been a retrenchment, investment levels are still historically quite high. The year 2021 was an exception, with levels of cash deployment that could never be sustained. Despite venture firms show strong investor interest in life sciences, a lengthy global slowdown will finally be felt.[127]
- The beginning of a new era: next-generation therapeutics, with the contribution of Dr Rino Rappuoli[128]
The frontiers of life science research and development have received extraordinary public attention over the past 12 months. The incredible progress made in the creation of COVID-19 vaccines, particularly mRNA vaccines, has elevated next-generation medicines to the forefront.
According to sell-side consensus estimates from Evaluate Pharma in June 2020 for the market for cell, gene, and nucleic acid therapies, it was expected to reach $38 billion over the following four years. With COVID-19 vaccinations excluded, the 4-year projection has increased to $41 billion.
Prior to COVID-19, next-generation medicines were already capturing attention.
Global COVID-19 vaccine sales were projected to reach an astounding $49 billion in 2021, with the majority of that amount ($46 billion) going to mRNA vaccines from Pfizer/BioNTech and Moderna.
Evaluate Pharma projects that the market for next-generation therapies will reach $59 billion in 2026, excluding vaccines. Gene therapy is predicted to increase more quickly than cell therapies, including regenerative methods, in the field of next-generation pharmaceuticals. The $20 billion gene therapy market is expected to be the largest next-generation market in 2026, with Novartis’ Zolgensma as the market leader.
In comparison to the same research conducted in 2020, Evaluate Pharma identified in 2021 about 6,500 active cell and nucleic acid therapeutic R&D programs, an increase of over 1,000 (20%). These growth rates represent a significant improvement over the equivalent comparison for 2019 to 2020, and they indicate that rather than slowing R&D, the COVID-19 era is marked by an acceleration of progress in the field of next-generation therapies. As a result, the entry of large pharma into the next-generation therapeutic area, notably through sizable deals for relatively early-stage drug developments, became a dealmaking theme for 2020. The extraordinary clinical and financial success of the mRNA COVID-19 vaccines serves as one example of how the next-generation pharmaceuticals environment is always breaking new ground.
- Relevant mRNA vaccine developments: infectious diseases and cancer
Infectious diseases
Researchers have developed vaccinations for the prevention and control of numerous infectious diseases since the first vaccine against cowpox was administered in 1796.[129] Inactivated microorganisms used in traditional vaccines have proven to be quite effective in preventing more than 30 infectious illnesses worldwide.
Traditional vaccines, however, still fall short of providing a high level of protection for several harder to eradicate infectious diseases. Strong humoral and cellular immune responses are elicited by mRNA vaccines, which represent a breakthrough in vaccine science. Several mRNA vaccines developed for difficult viruses like the human immunodeficiency virus (HIV), which cause recurrent or chronic infections, have also entered clinical trials. The promising outcomes of these applications highlight the significance of mRNA vaccines for the upcoming creation of vaccinations against infectious diseases.
Table 1: Representative mRNA vaccines in clinical trials for the prevention of infectious diseases[130]
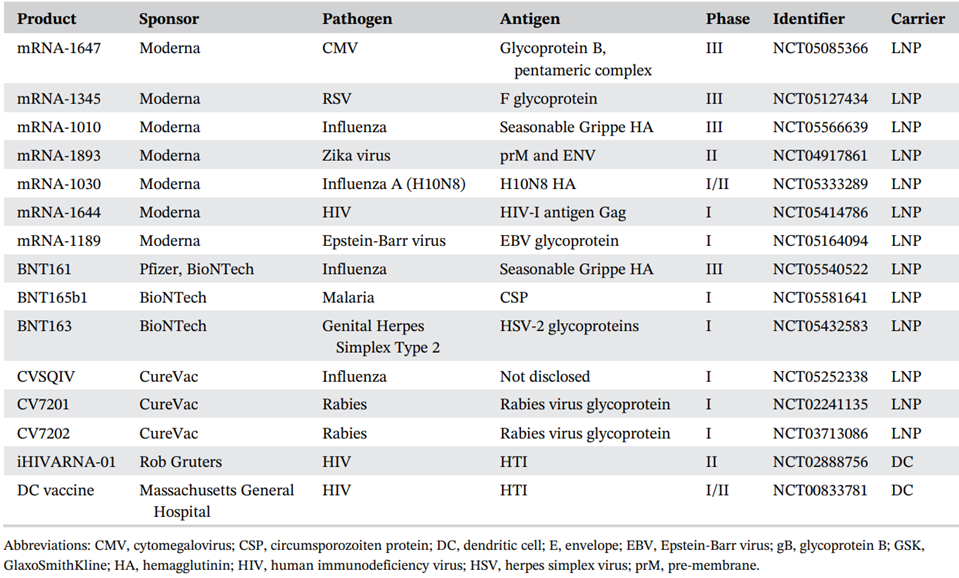
The threat of influenza viruses to human health continues to be seasonal. The influenza virus vaccine is typically multivalent and must be renewed each flu season due to the rapid viral mutation.[131] Strong immune responses can be elicited by mRNA vaccines, and they can also be quickly modified to target new variant antigens, indicating significant potential for the creation of effective influenza virus vaccines. Pfizer and BioNTech created BNT 161, a modified mRNA vaccine for the prevention of influenza, which is currently being examined in a Phase III study in the USA. Moderna has created two mRNA vaccines against the H10N8 and H7N9 influenza viruses, which have been demonstrated to be safe and immunogenic.[132]
The Zika virus and the Cytomegalovirus are other viruses that are active right now. An unaltered, encapsulated mRNA-1893 vaccine against Zika has been created by Moderna; it was quickly approved by the FDA and was undergoing phase I trials to determine its immunogenicity, safety, and tolerability.[133] Importantly, in a mouse model of congenital infection, mRNA-1893 stopped the virus from being transmitted during pregnancy.
Then, John et al.[134] developed a cytomegalovirus (CMV) vaccine to stop CMV infection and illness in transplant patients as well as during pregnancy. It has been tested in a clinical trial supported by Moderna (mRNA1647) and produced a potent immunological response in mice and non-human primates after a single dosage.
Because both humoral and cellular immunity are required to destroy the circulating virus and infected cells, it is more challenging to produce an efficient antibody response with a therapeutic impact for some chronic infectious viruses, such as HIV-1, hepatitis virus, and chikungunya virus.[135] Despite the fact that HIV was discovered more than 30 years ago, no effective vaccine has reached the clinical stage;[136] nonetheless, mRNA vaccines have demonstrated significant promise for the creation of an HIV vaccine. However, mRNA vaccines for HIV are still a long way from being used in clinical trials, and it may also be necessary to combine them with other therapeutic approaches for a more effective therapeutic outcome.
Hepatitis C virus (HCV) chronic infection could be controlled and treated with direct-acting antiviral treatments, in contrast to HIV infection without effective therapy. But no reliable HCV vaccinations have been created. However, there have been few documented preclinical or clinical tests using mRNA-based HCV vaccines. The public’s health is still threatened by several diseases, including bacteria, fungus, and parasites. In mice, mRNA vaccinations against malaria have recently been shown to be superior to conventional vaccines.[137]
Cancer
The idea of utilizing therapeutic vaccinations to treat cancer has been developed by researchers for decades. The goal of an mRNA tumor vaccine is to trigger a cell-mediated response, such as the conventional T lymphocyte response, in order to eliminate or reduce the number of tumor cells without damaging healthy cells.[138]
Despite the fact that cancer mRNA vaccines have made significant progress in the clinic, immunosuppression of the tumor microenvironment frequently limits vaccine-induced T-cell infiltration in tumor tissue, making them ineffective for treating advanced solid cancers. An effective way to treat cancers is to use the cancer mRNA vaccine in combination with other treatments including chemotherapy, checkpoint inhibitors, and immune agonists.[139]
Three business tycoons, Moderna, BioNtech, and CureVac AG, are dedicated to mRNA technology and have made significant investments in the use of mRNA technology in cancer vaccines. There are now several mRNA cancer vaccines in clinical studies.
Table 2: Representative mRNA-based vaccines in clinical trials for the treatment of cancers[140]
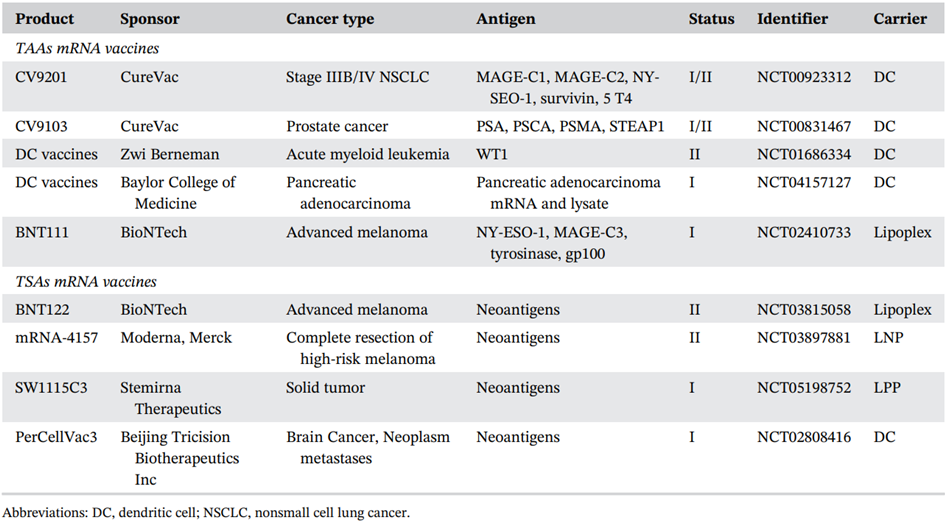
The mRNA-4157, a customized therapeutic vaccination for melanoma, is one of many neoantigen-encoded mRNA cancer vaccines that Moderna has developed against many types of cancer. Other anti-tumor vaccines being developed by BioNtech include BNT111 for advanced melanoma, BNT112 for the treatment of high-risk localized prostate cancer and metastatic castration against it, BNT114 for triple-negative breast cancer, and BNT115 for ovarian cancer.
A significant revolution in mRNA cancer vaccines has been brought about by the quick development of delivery mechanisms and neoantigens. The remarkable potential of individual cancer vaccines encoded by mRNA has been validated by accumulating results. However, there are still few responsive neoantigens found in clinical trials.
- The regulatory and legislative situation and perspectives, with the contribution of Dr Rino Rappuoli[141]
The issues surrounding current regulation and legislation, which arose during the Covid-19 pandemic, gave rise to a wide-ranging debate, which we discussed earlier in the dedicated section. What emerges is a contrast between two diametrically opposed ideas.
The first, more widespread and popular, supported by the plurality and some governments, is the one that questions the validity of intellectual property and patents that are directly involved in the study, development and production of medicines, particularly vaccines, and more generally of any life-saving product.
The second one, carried out by scientists, scholars and experts in the pharmaceutical sector and more specifically in vaccines, emphasises the importance of the existence of patents and rejects the opponents’ thesis that it is patents that are the main obstacle to the diffusion of new technologies and the related progress in the medical and vaccine field.
On this subject, in addition to the research carried out and set out above, we provide here the contribution and opinion of Dr Rino Rappuoli, a world-famous Italian microbiologist, Scientific Director of the Biotecnopolo Foundation of Siena, of which the National Anti-Pandemic Centre (CNAP) is also a member, scientific coordinator of the MAD Lab at Toscana Life Sciences in Siena, and winner of several awards in the medical and vaccination field, interviewed by candidate Alice Pizziconi, on June 6 2023.
These are his words on the subject:
“All those who argue that patents should be made freely available don’t understand the system. It’s a pretty easy way for politicians to blame someone else, but they really don’t understand what’s going on in the world. It’s a very popular way of looking at it, it’s easy to say: “it’s the patents’ fault”. But patents have nothing to do with it. Those who wanted to use this to shift the blame on companies and patents didn’t say: “now we will pay our royalties, maybe we will discuss the price because for developing countries that standard would be too high. But we will respect your rights to obtain the concession and use them”. They said: “we will obtain freely the patents because the patents are not valid anymore”. It’s like going into someone else’s house saying: “I need a place to stay, you get out of here and I’ll take your house”. It’s not OK, it’s stealing…
It’s called intellectual property, if someone says: “give it to me”, it has to be negotiated, it can’t be stolen. And that is an argument. Then, there is a lack of expertise to develop and produce them. There is a lack of know-how, and this is the reason why mRNA vaccines have not been developed and produced in developing countries. You could give them all the patents in the world, they would not produce half a dose. Neither would they today. So, this line of reasoning is nothing more than a misleading and simplistic way of looking at the issue, blaming things that don’t have any, and unfortunately it is very easy then for the plurality, in the absence of all the facts of the case, to simply adhere to that thinking.”[142]
And again:
“There is a beautiful example that was given, because these debates always born when there are injustices between developed and developing countries. It had already happened with HIV drugs. When those drugs were allowing people in the western world to survive in the late 1990s, while millions were dying in Africa and elsewhere, the question became: ‘the problem here is that there are patents on the drugs’. So, the US government started a programme called PEPFAR, to make 15 billion available, not to give to those who had the patents, but to give chemical companies in developing countries a chance to learn how to make those drugs. In doing so, the companies that had patents on the drugs agreed to give these chemical companies the opportunity to produce them without paying royalties or with minimum prices, provided, however, that they produced them for developing countries and did not in any way interfere with the market in western countries. This system still works, and it does not provide for universal patenting. Thus, there are quite simple solutions, which have nothing to do with patents, but which have as a basic prerequisite, bringing to companies that are in developing countries the ability to be able to do these things and transfer the technology.”[143]
What emerges, therefore, is a need for greater clarity on the real, albeit complex, system regulating the development and production of vaccines. It is not the abolition of intellectual property that is the solution, rather a reorganisation of this system, providing the know-how first. The focal point, however, remains respect for intellectual property and the protection of the companies that exercise it, this to protect not only the companies themselves, but above all the investors, private and public, that support them economically. Investors are essential for the progress of research and the subsequent development of new technologies. If there is no primary protection for such investments, they have no reason to be made, and we know that without investment there can be no innovation. A huge damage to the progress of research, which harms everyone.
- The economic highlights of this new technology, with the contribution of Dr Rino Rappuoli
The analysis of the economic impact of the new mRNA technology looks more complex. As the pandemic has just ended, it is not yet possible to obtain a clear picture of the effects of the new technology on the markets and the pharmaceutical sector. However, there are several interesting aspects that are worth studying more deeply, thanks to the contribution of Dr Rappuoli.
The first topic discussed with the professor concerns the foundation of research and innovation: investment. Several countries, during the pandemic, invested heavily in research. Let’s see what their impact on the development of these vaccines has been and how much they can also contribute to the development of future vaccines:
“Clearly, investments were essential to produce the vaccines in 10 months. To make them so quickly, to have so many millions of doses. Without those investments, technology alone would not have made it possible to make vaccines so quickly, so the investments are indispensable, because otherwise the technologies would not develop, the vaccines would not be made so quickly. It is clear that in this case there was a lot of investment. Operation Warp Speed provided 12.5 billion, but Europe has also spent a lot of money, England, Japan. These are public sector investments that then essentially came back to the public. So, I find them very appropriate investments, which unfortunately were made on a one-off basis, because there was a pandemic, instead they should be made often, continuously, in order to bring more technology for the benefit of human beings.”[144]
When asked about the current state of investment, quantity and frequency, given the end of the pandemic and the likely decrease in interest in this technology, the professor continues:
“The interest in investing has already decreased a lot. All the goodwill, all the good intentions that were there have waned after the pandemic, gone almost to zero, for several factors. First, because the pandemic is over and with it the relative urgent need, then because other priorities have arisen, such as the war, and so now we talk about other things. The pandemic is no longer an emergency and so essentially the enthusiasm to invest has waned so much. I hope it won’t go back to the zero level it was before, but yes, it has decreased a lot.”[145]
Another topic discussed with Dr Rappuoli concerns the price of future mRNA vaccines, which is expected to be particularly high and therefore potentially not accessible to all, although in reality it has been said several times that this type of vaccines is not only faster to produce, but it is also less expensive than some traditional vaccines. The main reason would seem to be the investments that companies, especially after the successes achieved during the pandemic, have made for the development of additional mRNA vaccines. On this topic, Rappuoli is expressed as follows:
“When you look at prices people always try to do their proper counts, they basically do their own maths. How much does one component cost, how much does the other component cost, and how much do you make compared to components that you use. Nobody considers that from 1993 to 2020 there were private individuals and the public sector that invested for almost thirty years in technologies that until then had achieved nothing. And this is money that private individuals, all those who invested, risked losing. The investors of Moderna, of CureVac, of BioNTech, of Novartis. Decades of investment for a technology that after many years has become useful. And when you think about the cost of an innovative product you must bear in mind that those who have invested all these years somehow need a return on investment, otherwise nobody would invest in innovation anymore. So, in my opinion doing the maths on cost and return is not wrong. If we want to incentivise innovation, we have to pay a price that then repays the investment that has been made for decades in research and development.”[146]
To conclude the analysis of the main factors directly related to the economic sector, it has been discussed with Dr Rappuoli the topic of big Pharma and the patents in their possession, also considering the strategic acquisitions made during the pandemic to encourage the development and then the production of vaccines. All these elements give big Pharma’s considerable power and influence, which over time have led many to wonder whether this could then result in the creation of oligopolies or even monopolies. Rappuoli has no doubts about it:
“My view is that there will not be an oligopoly because of patents. There might be an oligopoly because Moderna, BioNTech and Pfizer started earlier than the others and therefore have more innovative products, they will produce the next most innovative products first and therefore they will be market leaders for that. But surely the risk of oligopolies cannot be attributable to patents. Why do I say this? Because there are more than 20,000 patents on RNA. Moderna cannot operate alone, it obtains under licence the patents. Pfizer obtains under licence the patents. BioNTech obtains under licence the patents. CureVac obtains under licence the patents. Everyone who works on RNA will obtain under licence and then use some of those 20,000 patents. So, the only way I think the vaccines of the future will be developed and produced is the way you build a car today. There is no patent on the car. So, there is nobody who can establish a monopoly because nobody owns that patent. Whoever makes a car buys a component, like brakes, from someone who has patents on brakes. The electronic component is becoming a very important part, so you buy the electronic component from someone who owns that patent, the wheels from someone else, the rims from someone else, and so on. And then you put it all together, paying royalties to all these different companies, to build the car that we then pay for. I think RNA will become something like that. Everyone more or less will be able to use it, and everyone will pay royalties. Moderna will pay royalties to the patents they request, maybe they will pay less because they have many patents themselves. Pfizer will do the same. CureVac has a big patent portfolio, and then there are many other patents. With 20,000 patents, you realise that it is impossible to do things on your own, independently; so no, I don’t think patents will ever be an obstacle to the distribution and commercialisation of RNA products.”[147]
The elements that have been considered and the issues dealt with Dr Rappuoli can be traced back to a short-term analysis on some effects of mRNA technologies on the economy. It is clear that a long-term analysis will have to wait a few more years, to see how markets, particularly pharmaceuticals, will evolve as a result of the numerous revolutionary changes that have occurred during the pandemic.
- Future challenges and opportunities for the mRNA vaccines, with the contribution of Dr Rino Rappuoli
The successful application of a COVID-19 vaccine to stop the global spread of SARS-CoV-2 has raised optimism that a larger array of vaccines for the prevention of infectious diseases and the treatment of cancer will be created using the same technology. However, there are still a number of concerns with the mRNA molecule and delivery systems that need to be resolved for further development, including issues with immunogenicity and stability, biosafety, targeting, and large-scale mRNA vaccine production.
The most difficult problems in the creation of efficient vaccines continue to be novel pathogen infections and immune escape of tumors caused by the mutation of targeted antigens.[148] Particularly with RNA viruses, infectious diseases may create various levels of mutation rates throughout transmission.[149]
Comparatively speaking, the processes for pathogen breakthrough infections are much simpler than those for immune escape of cancer.[150] The main causes of the complexity are thought to be immunosuppressive tumor microenvironment and the loss or mutation of targeted antigens. Cancer vaccines have entered a new era thanks to the identification of neoantigens, but as these neoantigens can suffer further mutations and be altered by immunoediting processes, their therapeutic efficacy may be diminished or even lost.[151]
Another problem to face is the stability. Since vaccine integrity is extremely sensitive to temperature, it is crucial for their effectiveness that they are stored and transported within a suitable temperature range from the time of manufacturing until the time of administration. For mRNA vaccines, the requirement for such cold storage still poses a problem. The LNP-mRNA system’s instability can be related to the severe temperature requirements for mRNA vaccine storage. The need for such extreme cold chain storage and transit of mRNA vaccines remains unsolved, and this could have a significant impact on future large-scale mRNA vaccine uses.
Synthesis, purification, and formulation are the three primary phases in the production of mRNA vaccines; each step additionally includes a number of sub steps. All of these processes, however, were not developed using a continuous production process. The production of mRNA vaccines might be substantially more efficient with a continuous manufacturing approach. For flexible and economical manufacturing, this method is already employed in the chemical and pharmaceutical industries.[152] It might be a viable plan for producing mRNA vaccines in big quantities and at a reasonable price.
The comparatively increased frequency of side effects compared to those brought on by conventional inactivated vaccinations, particularly for grade 3 adverse responses, is a final issue with mRNA vaccines. The lack of consistent and comprehensive international or national quality control standards may cause variations in the physicochemical characteristics and efficacy of mRNA vaccines produced by various producers.[153] Additionally, there is a risk of contamination due to the limitations of purifying techniques and the lack of very sensitive impurity testing tools.[154]
Despite the multiple challenges to face, mRNA vaccines exhibit significant advantages over other vaccinations due to their capacity to induce both humoral and cell-mediated immune responses, quick antigen design flexibility, and use of the same manufacturing platform for several mRNA vaccines. There are many different kinds of mRNA vaccines that have been created, such as therapeutic vaccines against cancer and preventive vaccines against infectious diseases.
The fact that mRNA vaccines have worked well and have been developed, produced, approved and distributed much more quickly than traditional types of vaccines and also in general compared to what are normally the timeframes, has generated great expectation and hope among the population all over the world about the future of vaccines, especially in relation to viral diseases and cancers, which Dr Rappuoli indicated as the reason why these platforms were born and were being developed. I asked him if this technology can be considered as a definitive turning point in the world of vaccines and the progress it is achieving.
“Surely this is a new, revolutionary technology. Revolutionary because it is the first completely synthetic vaccine that has been made, because it is made from messenger RNA, which is a four-letter chemical code, not very different from the binary code 01, and it is the first time a vaccine has been synthesised completely from the beginning, all synthetic, and injected. We then specify that what is injected is not a vaccine, but the information with which our cells then produce the vaccine. So, it is a transmission of information. This technology is not new. I think the first time it was shown that RNA could be used was in the early 90s, between 92 and 93 to be precise, then in 2000 the first biotechnology company CureVac came along. Then came Moderna in 2010/11, then BioNTech and so on. By 2020 this technology had made a lot of progress, had been in clinical trials in a few limited cases, but was not ready to be developed. If there wasn’t a pandemic, we would have had RNA registered for some product probably in 2028, in 2030, because it didn’t have the robustness that we generally expect from drugs. The first thing we had to ensure was stability, we had to store vaccines at -80°, under normal circumstances nobody would ever develop a vaccine that is kept at -80°. Then there is the reactogenicity, which is still too high, this type of vaccine is very reactogenic compared to other types; all this to say that it was a new technology but not ready to be developed. When the pandemic arrived, the cost/benefit of the risk accelerated the clinical use and the use in billions of people of this technology. And this has on the one hand saved the people who have been vaccinated, and on the other accelerated the new RNA technology, which was used for Covid vaccines, but is now being exploited for many other vaccines. There is the Cytomegalovirus vaccine that is in phase III by Moderna, there are vaccines against influenza, vaccines against respiratory syncytial virus. There are so many vaccines that are coming faster than expected, because this technology has now been approved, and thanks to the pandemic it has accelerated by 7/8 years. This technology has so far done nothing that other technologies did not do. Let me explain, all the Covid vaccines made with different technologies have worked; therefore, RNA has not been anything special in that sense. What was special was the speed. The speed with which it was developed and the speed with which it was produced in hundreds, thousands, billions of doses, which with more conventional technologies would have been much more difficult.” [155]
“So, when we look at the future of RNA we have to consider that RNA is not just vaccines, in the future we will have a new class of full spectrum drugs that will be made with RNA. These promises will come true, they will come faster than they would have come without the pandemic clearly, and we already have a first resounding example, which is the melanoma vaccine that has been announced via Press release. For now, there is no official document, but the data was shown at a congress, it is the melanoma vaccine announced by Moderna and Merck. This is a fully customised vaccine. Patients who had their tumour surgically removed were divided into two groups, one group was given the conventional therapy, that is already very good for melanoma, which is pembrolizumab, commonly known as Keytruda from Merck. And the other group was given Keytruda together with a customised vaccine, different for each patient. The vaccine contained the sequences of 34 tumour-specific epitopes for each patient. Each patient was given the genome of the healthy tissue and the genome of the tumour tissue, once the differences were found, 34 epitopes specific to that person’s tumour were encoded in the unique messenger RNA that was the vaccine. And from what was announced during the press release, this vaccine had a 44% improvement on recurrence compared to people who were treated with Keytruda alone. This is an incredible result, but the most important thing is that this is the first cancer vaccine after a century of failures that proves to work for cancer treatment. So clearly this is the first demonstration that vaccines also work against tumours. If we are breaking new ground, it is thanks to RNA and tumour vaccines based on this technology.”[156]
To conclude, Dr Rappuoli discussed the significant differences between the cancer vaccines that are currently available and those that this technology will produce. He did this to clarify why there is currently much more enthusiasm for mRNA platforms than for cancer vaccines that are currently available.
“There is a fundamental difference between the two types of vaccines. We have since the early 1990s a vaccine that prevents liver cancer, which is caused by the hepatitis B virus. All newborns in the world are vaccinated, there will be millions of cancers prevented thanks to this vaccine. Since 2006 there is the vaccine against papilloma virus that prevents cervical cancer. This vaccine is unfortunately not used in developing countries, so there are about half a million deaths a year from cervical cancer there. These are real cancer vaccines, because they are eliminating these cancers from the world. These vaccines prevent the cancer, so people will never get it. These vaccines, unfortunately, are undervalued for their anti-cancer approach, because thanks to them people are no longer affected, so they tend to take this immunity for granted. It is like the vaccines against smallpox, which killed 300 million people last century. Now smallpox is no more. People today have forgotten about this disease. Instead, the mRNA vaccines we talked about earlier, are therapeutic vaccines. These will save very few people, these people will die anyway, for the most part. They will only live a bit longer. But they have an enormous impact both economically and personally, because when you have a tumour, you are desperate, and willing to pay whatever it takes, you no longer think, and you put everything you can on the table to get something that can improve your life. And this is, unfortunately, essentially human nature. When we are well, we never think, we never consider how important it is to prevent, when we are sick it is clearly very important to treat ourselves, and rightly so. But from an economic point of view, it has to be said why the therapeutic cancer vaccines for which mRNA vaccines have been developed have had a lot of support from investors, venture capital… because on cancers, on cancer treatments, there is a lot of money to be made. It’s not fair, but there is.”[157]
Conclusions
Deep transformational processes were already having an impact on the global pharmaceutical industry prior to the pandemic-induced catastrophe. In a highly globalized sector of the economy with extensive, articulated supply chains, trade tensions and a trend toward regionalization of trade have raised concerns about the need to rethink organizational structure, especially in light of new technologies and the accelerating pace of digitalisation.
This process has been enhanced as a result of the critical issues that surfaced during the lockdown, including the disruption of logistical supply chains and the shortage of drugs and medical devices in many advanced economies. This has highlighted the strategic relevance and importance of a healthcare sector that can react quickly and comprehensively to emergency situations.
We analysed various aspects related to vaccines, particularly in the context of the COVID-19 pandemic. We also provided information on the supply chains and manufacturing processes of various COVID-19 vaccines and the profits made by pharmaceutical companies. The use of nucleic acid therapies, such as mRNA, has shown promise in vaccine development. We discussed the development and production of mRNA vaccines, which use messenger RNA to instruct cells to produce a specific protein that triggers an immune response. We also highlighted the challenges of scaling up production of these vaccines, particularly the difficulty in obtaining sufficient quantities of lipids, which are used to protect the mRNA molecules. The logistics of vaccine distribution and the expense of cold-chain storage are also significant challenges in the global vaccination effort. We provided information on the supply chains and manufacturing processes of various COVID-19 vaccines, including Pfizer/BioNTech, Moderna, AstraZeneca/Oxford University, and Johnson & Johnson/Janssen. We talked about the partnerships and collaborations between these companies and various contract development and manufacturing organizations (CDMOs) to produce and distribute the vaccines globally.
The regulatory and intellectual property debates surrounding vaccines have been deeply analysed. We discussed the patent landscape for COVID-19 vaccines and the balance between protecting intellectual property rights and ensuring access to life-saving medications.
The Italian patent in the pharmaceutical industry was legitimized by the Constitutional Court only forty years ago, making it far from a legislative effort. The government was given the authority to require patent holders of drugs and vaccines to grant their non-exclusive use to the state or third parties in the event of a health emergency. The Marrakesh Agreement, which led to the creation of the World Trade Organization, established the protection of intellectual property rights as a means of advancing technological innovation. However, the TRIPs Agreement also allows for exceptions and restrictions to these exclusive rights, including compulsory licensing for pharmaceutical products during public health emergencies. The Covid-19 pandemic has brought attention to the issue of patent protection for vaccines and medical technologies, with some countries proposing a waiver of TRIPs provisions.
Intellectual property has always been a sensitive and complex subject, especially in relation to a sector such as pharmaceuticals, which is directly related to health. When we talk about medicines and vaccines, an additional element comes into play, which for other sectors does not emerge: ethics. It is true that intellectual property and patents are essential for the protection not only of big Pharma but also of investors, and therefore indirectly, of innovation, but we also take into account the fact that the mRNA platforms, because of their enormous potential and their nature, should be able to be best exploited by all.
From an economic perspective, mRNA vaccines have several key implications and considerations. Developing mRNA vaccines requires substantial investments in research and development (R&D). Costs can be significant, but they are often mitigated by government funding, public-private partnerships, and philanthropic support. In the case of COVID-19 vaccines, governments and international organizations provided substantial financial resources to accelerate the development and production of mRNA vaccines. Scaling up the production of mRNA vaccines can be a complex and expensive process. Compared to traditional vaccine manufacturing methods, mRNA vaccines have unique production requirements, including the need for specialized facilities and technologies. Building and modifying manufacturing infrastructure to produce mRNA vaccines at a large scale can entail substantial costs. However, mRNA vaccines offer potential advantages in terms of flexibility and adaptability, allowing for rapid adjustments to address new variants or emerging diseases. Pricing strategies for mRNA vaccines can vary depending on factors such as manufacturing costs, supply and demand dynamics, negotiations with governments and purchasers, and intellectual property considerations. The pricing of mRNA vaccines has been a topic of debate, with concerns about ensuring global access, particularly for low- and middle-income countries. Beyond the immediate pandemic response, mRNA vaccine technology has the potential for broader applications in healthcare. The versatility of mRNA platforms allows for the development of vaccines targeting various infectious diseases and potentially even therapeutic interventions for cancer and other conditions. Exploring these applications can have long-term economic implications, including advancements in public health, reduced disease burden, and potential cost savings. The pharmaceutical industry is one of the most profitable business sectors in the world, with pharmaceutical companies expanding their cash reserves and increasing dividend payouts to shareholders. Pharmaceutical companies are spending billions of dollars to acquire rival businesses to stifle competition, expand their patent portfolio, and develop new medication pipelines. The COVID-19 vaccine companies, including Pfizer/BioNTech, Moderna, Sinovac, and AstraZeneca/Oxford University, are listed on stock exchanges, and their shareholders have received a sizable chunk of the gains.
The enthusiasm generated by the successes achieved by mRNA vaccines makes hope for a revolution in the world of vaccines, which will arrive much earlier than expected, as pointed out by Dr Rappuoli during the interview. The future of mRNA technology holds significant promise and potential across various fields of medicine and beyond. mRNA vaccines have already proven their effectiveness in combatting infectious diseases. In the future, mRNA technology is likely to play a crucial role in developing vaccines against other infectious diseases. The ability to rapidly design and produce mRNA vaccines offers the potential to address emerging pathogens and adapt to new variants quickly. mRNA technology has the potential to revolutionize the field of therapeutics by enabling the development of novel treatments for a wide range of diseases. mRNA therapeutics can provide precise and customizable instructions to cells, directing them to produce therapeutic proteins, antibodies, or other desired molecules. This approach could be applied to various conditions, including cancer, genetic disorders, autoimmune diseases, and rare diseases. This technology could facilitate the development of personalized medicine approaches. The customizable nature of mRNA allows for tailoring therapies to an individual’s unique genetic makeup, enabling targeted treatments. This could lead to improved treatment efficacy, reduced side effects, and better patient outcomes. Finally, mRNA-based cancer immunotherapies are being explored as a promising approach. By encoding tumor-specific antigens into mRNA, personalized cancer vaccines can be developed to stimulate the immune system and target cancer cells.
It is important to note that while mRNA technology holds significant promise, there are still challenges to address, such as optimizing delivery systems, ensuring long-term stability of mRNA therapeutics, and furthering our understanding of immune responses. Nonetheless, the ongoing advancements and research in mRNA technology are likely to pave the way for transformative breakthroughs in medicine and various other fields in the future.
However, given these facts and the significant growth potential of this ground-breaking technology, the question of how the problem of intellectual property will affect mRNA platform advancements in the future arises. Again, given the detailed and incredibly complex contemporary landscape, what will be the long-term effects of this new technology on the pharmaceutical industry and, especially, the vaccine sector?
References
- Abdel-Hakeem, M.S., Shoukry N.H., Protective immunity against hepatitis C: many shades of gray, National Library of Medicine, Front. Immunol., 2014
- AboulFotouh, K., Cui, Z. and Williams III, R.O., Next-Generation COVID-19 Vaccines Should Take Efficiency of Distribution into Consideration, AAPS PharmSciTech. 2021
- Advanced Gene-based Vaccines along with the Advent of the Pandemic boomed the Vaccine Market: Analysis by FMI for 30+ countries, FutureMarketInsights, 2023 https://www.futuremarketinsights.com/reports/vaccines-market
- Agenzia Italiana del Farmaco (AIFA), Covid-19, Vaccines https://www.aifa.gov.it/vaccini-covid-19
- Aljadir, A.; Alnemsh, M. Exploration of the COVID-19 pandemic in relation to the healthcare industry Supply Chain 2020
- Almurisi, S.H., Khalidi, D.A., AL-Japairai, K.A., Mahmood, S., Chilakamarry, C.R., Kadiyala, C.B.N. and Mohananaidu, K., Impact of COVID 19 Pandemic Crisis on the Health System and Pharmaceutical Industry, Researchgate, 2021
- Annual and quarterly reports of the seven companies. Biomedical Advanced Research and Development Authority (BARDA), COVID-19 Medical Countermeasure Portfolio
- Armbruster, N., Jasny, E. and Petsch, B., Advances in RNA vaccines for preventive indications: a case study of A vaccine against rabies, MDPI open access journals, 2019
- Arnaudo L., Pitruzzella G., La cura della concorrenza. L’industria farmaceutica tra diritti e profitti, Roma, 2019
- Arvinas and Pfizer Announce Global Collaboration to Develop and Commercialize PROTAC® Protein Degrader ARV-471, Pfizer, 22 July 2021
- AstraZeneca, Annual Report and Form 20-F Information for 2020 and AstraZeneca, Annual Report and Form 20-F Information for 2021
- Beatty, G.L., Gladney, W.L., Immune escape mechanisms as a guide for cancer immunotherapy, National Library of Medicine, Clin. Cancer Res., 2015
- Biomedical Advanced Research and Development Authority (BARDA), COVID-19 Medical Countermeasure Portfolio
- BioNTech, 20-F Annual report 2021, filed at SEC, March 2022
- BioNTech, 6-K report third quarter 2022, filed at SEC, 7 November 2022
- BioNTech, Share Repurchase Program, https://investors.biontech.de/share-repurchase
- Biontech.de, The role of mRNA from the vaccine https://impfung.biontech.de/it/impfung/mechanism-of-mrna.html
- Biopharma and Medtech Review 2022, Evaluate Pharma, 2023
- Biopharma and Medtech Review 2022, Evaluate Vantage, 2023
- Biopharma and Medtech Review 2022, Evaluate, 2023
- CEPI statement: CEO welcomes Emergency Use Listing for NVX-CoV2373, CEPI, 17 December 2021
- Council for Trade-Related Aspects of Intellectual Property Rights, WHO IP/C/W/ 669, 2.10.2020
- COVAX Working Group on delivery costs, Costs of delivering COVID-19 vaccine in 92 AMC countries, section 5.2, table 9, 2021, Costs of delivering COVID-19 vaccine in 92 AMC countries (who.int)
- COVID-19 guidance: evaluation and marketing authorisation, European Medicines Agency https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/guidance-developers-companies/covid-19-guidance-evaluation-marketing-authorisation
- Cullinal, K., Wealthy Countries Urged to Share 2 Billion Excess COVID-19 Doses, as South Africa’s mRNA Hub Prepares to Make ‘Moderna-like’ Vaccine, Health Policy Watch, 2021
- Dakin, J., Supply Chain Challenges Creating Hurdles to COVID-19 Vaccine Production, Pharmtech, 2021
- De Haan, E. and ten Kate A., Pharma’s Pandemic Profits, Pharma profits from COVID-19 vaccines, SOMO, 2023
- Decreto legge 7 giugno 2017, n. 73 https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=59548
- Diamond, D.J., Rosa, C.L., Chiuppesi, F., Contreras, H., Dadwal, S., Wussow, F., Bautista, S., Nakamura, R. and Zaia, J.A., A fifty-year odyssey: prospects for a cytomegalovirus vaccine in transplant and congenital infection, Expert Review of vaccines, Volume 17, 2018
- Dolgin, E., The tangled history of m RNA vaccines, Nature, 2021
- Dolgin, E., Unlocking the potential of vaccines built on messenger RNA. The technology could help to boost immunity against cancer, influenza and much more, Nature, 2019
- Duffy, S., Why are RNA virus mutation rates so damn high?, PLOS Biology, 2018
- EMA, Biosimilar medicines: overview, https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview
- EMA, Transfer of marketing authorisation: questions and answers, Transfer of marketing authorisation: questions and answers | European Medicines Agency (europa.eu)
- European Commission, Safe Covid-19 vaccines for Europeans, https://commission.europa.eu/strategy-and-policy/coronavirus-response/safe-covid-19-vaccines-europeans_en
- European Medicines Agency, human regulatory, Covid-19 https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-development-evaluation-approval-monitoring
- European Medicines Agency, Studies for approval https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-studies-approval
- Farmaceutico e Covid-19: Alcuni fatti stilizzati, Andrea Montanino, Simona Camerano, Alberto Carriero, Angela Cipollone, Roberto Giuzio, CDP, 2020.
- Farmindustria, Indicatori farmaceutici, 2022
- Fernandez, R. and Klinge, T. J., The financialisation of Big Pharma, SOMO, 2020
- Galli, C., Il diritto della proprietà intellettuale di fronte alle sfide della pandemia, in Dir. ind., 2021
- Garde, D. & Saltzman, J. – The story of mRNA: How a once-dismissed idea became a leading technology in the Covid vaccine race. Stat, 2020
- Gaviria, M. and Kilic, B., BioNTech and Pfizer’s BNT162 Vaccine Patent Landscape, Public Citizen, 2020
- Gaviria, M., Kilic, B. A network analysis of COVID-19 mRNA vaccine patents. Nat Biotechnol.
- Geneva Graduate Institute, Global Health Centre, COVID-19 Vaccines R&D Investments, 2021
- German Federal Ministry of Education and Research, Coronavirus: What the BMBF is doing, 2020
- Gilles, C., Greeley, B. and Arnold, M., Financial Times: Global recession already here, say top economists, 2020
- Governments spent at least €93bn on COVID-19 vaccines and therapeutics during the last 11 months, Business wire, 11 January 2021
- Grisoli A., La secolare ed incompiuta vicenda della brevettabilità dei farmaci, in Riv. dir. ind. 1981
- Harding, A.T., Heaton, N.S., Efforts to improve the seasonal influenza vaccine, National Library of Medicine, Vaccines, 2018
- Heilweil, R., The key ingredient that could hold back vaccine manufacturing, Vox, 2021
- Hill, A.; Wang, J.; Levi, J.; Heath, K.; Fortunak, J. Minimum costs to manufacture new treatments for COVID-19, Journal of Virus Eradication, 2020
- Hopkins, J.S., Eastwood, J. and Moriarty, D., mRNA Covid-19 Vaccines Are Fast to Make, but Hard to Scale: complex steps of new type of vaccine create production bottlenecks, The Wall Street Journal, 2021
- Indicative timelines for COVID-19 vaccines compared with standard vaccines, European Medicines Agency, Covid 19https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-development-evaluation-approval-monitoring
- Istituto di ricerche farmacologiche Mario Negri IRCCS, mRNA vaccines https://www.marionegri.it/magazine/vaccini-a-mrna
- Istituto Superiore di Sanità, epidemiology for public health, vaccines and immunisation https://www.epicentro.iss.it/vaccini/
- Istituto Superiore di Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/covid-19-vaccino-pfizer-biontech
- Istituto Superiore di Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/covid-19-vaccino-moderna
- Istituto Superiore di Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/covid-19-vaccino-astrazeneca
- Istituto Superiore di Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/covid-19-vaccino-janssen
- Istituto Superiore Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/VacciniCosaSono
- Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use – First Single-Shot Vaccine in Fight Against Global Pandemic, 2021 Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use | Johnson & Johnson (jnj.com)
- King, A., Why manufacturing Covid vaccines at scale is hard, ChemistryWorld, 2021
- Kose, N., Fox, J.M., Sapparapu, G., Bombardi, R., Ennekoon, T.R.N., Silva, A.D.D., Elbashir, S.M., Theisen, M.A., Narayanan, E.H., Ciaramella, G., Himansu, S., Diamond, M.S. and Crowe, J.E., A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection, Scopus, Science Immunology, 2019
- Kretchy, I.A.; Asiedu-Danso, M.; Kretchy, J.-P. Medication management and adherence during the COVID19 pandemic: Perspectives and experiences from low-and middle-income countries. Research in Social and Administrative Pharmacy 2020
- LeClair, D.A., Cranston, E.D., Lichty, B.D., Xing, Z., Thompson, M.R., Consecutive spray drying to produce coated dry powder vaccines suitable for oral administration, ACS Biomater Sci Eng. 2018
- Ledley, F. D., McCoy, S. S., Vaughan, G., Cleary, E. G., Profitability of large pharmaceutical companies compared with other large public companies, JAMA, 2020
- Legge 31 luglio 2017 , n. 119 https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=60201
- Li, L., Goedegebuure, S.P., Gillanders, W., Cancer vaccines: shared tumor antigens return to the spotlight, Signal Transduction Targeted Therapies 5, article n. 251, 2020
- Liu, T., Liang, Y., Huang, L., Development and delivery systems of mRNA vaccines, National Library of Medicine, Frontiers in Bioengineering and Biotechnology, 2021
- Lopez Angel, C.J., Tomaras, G.D., Bringing the path toward an HIV-1 vaccine into focus, National Library of Medicine, PLOS Pathogens, 2020
- Maffioli, E.M. In che modo il mondo sta rispondendo alla nuova malattia da coronavirus (COVID-19) rispetto all’epidemia di Ebola dell’Africa occidentale del 2014? L’importanza della Cina come attore nell’economia globale, Am. J. Trop. Med. Hyg., 2020
- Mallory, K.L., Taylor, J.A., Zou, X., Waghela, I.N., Schneider, C.G., et al., Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice, National Library of Medicine, NPJ Vaccines, 2021
- Martin, E., Bloomberg, Coronavirus economic impact ‘will be severe,’ at least as bad as Great Recession, says IMF, Fortune, 2020
- Massimiliano F., Vaccini, Brevetti e Big Pharma tra profitto, sostenibilità e diritto alla salute, in Dir. ind., 2021
- Miller, J., ‘Moderna Vaccine to Criss-Cross Continent before Europeans Get Shots’, Reuters, January 6, 2021
- Moderna reports third quarter 2022 financial results and provides business updates, Moderna, 3 November 2022 and Moderna 2Q 2022 Earnings Call, Moderna, 3 August 2022
- Moderna, 10-K Annual report 2021, filed at SEC, 25 February 2022
- Moderna, 10-Q report third quarter 2022, filed at SEC, 3 November 2022
- Moderna, ESG Day, 10 November 2022
- Moderna, Patents and Intellectual Property, Moderna’s Patents and pending patent applications (modernatx.com)
- Moriya, T., Kitagawa, K., Hayakawa, Y., Hemmi, H., Kaisho, T., Ueha, S., Ikebuchi, R., Yasuda, I., Nakanishi, Y., Honda, T., Matsushima, K., Kabashima, K., Ueda, M., Kusumoto, Y., Chtanova, T., Tomura, M., Immunogenic tumor cell death promotes dendritic cell migration and inhibits tumor growth via enhanced T cell immunity, iScience volume 24, 2021
- Nicholson Price II, W., Rai, A. K., and Minssen, T., Knowledge transfer for large-scale vaccine manufacturing, Researchgate, 2020
- Novavax Inc., Annual report 2021 including 10-K form, filed at SEC, March 2022
- Novavax, Investor presentation, January 2023, https://novavax.widen.net/s/bsldbcxbxh/novavax_corporate_investor_ deck_2023-01-09
- Ozili, P.K.; Arun, T. Spillover of COVID-19: Impact on the global economy, SSRN Electron. J. 2020
- Pancevski, B., BioNTech Recruits Rivals to Boost Covid-19 Vaccine Production, The Wall Street Journal, 2021
- Pardi, N., Hogan, M., Porter, F. & Weissman, D. – mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery 17, 261-279, 2018.
- Pfizer and Beam enter exclusive multi-target research collaboration to advance novel in vivo base editing programs for a range of rare diseases, Pfizer, 10 January 2022
- Pfizer, 10-Q report third quarter 2022, filed at SEC, 9 November 2022
- Pfizer, 20-F Annual report 2021, filed at SEC, February 2022
- Pfizer, 8-K report earnings release 2022, filed at SEC, 31 January 2023
- Pfizer, Proxy Statement for 2022 Annual Meeting of Shareholders, Summary Compensation Table, 28 April 2022,
- Pfizer, Third Quarter 2022 earnings teleconference, 1 November 2022
- Randewich, N., Coronavirus, oil collapse erases $5 trillion from U.S. stocks, Reuters, 2020
- Rappuoli, R., Personal communication, interview, June 6, 2023
- Roces, C.B., Lou, G., Jain, N., Abraham, S., Thomas, A., Halbert, G.W. and Perrie, Y., Manufacturing Considerations for the Development of Lipid Nanoparticles Using Microfluidics, MDPI Pharmaceutics, 2020
- Rosa, S.S., Prazeres, D.M.F., Azevedo, A.M., Marques, M.P.C., mRNA vaccines manufacturing: challenges and bottlenecks, National Library of Medicine, Vaccine, 2021
- Rosenthal, R., Cadieux, E.L., Salgado, R., Bakir, M.A., Moore, D.A., et al., Neoantigen-directed immune escape in lung cancer evolution, National Library of Medicine, Nature, 2019
- Rusnock, A., Catching cowpox: the early spread of smallpox vaccination, 1798–1810, National Library of Medicine, Bull Hist Med., 2009
- Rutschman, A.S., IP Preparedness for Outbreak Diseases, UCLA L. REV., 2018
- Rutschman, A.S., The Vaccine Race in the 21st Century, 61 ARIZ. L. REV. 2019
- Sadler, R., Who are the top suppliers in the Lipid market?, CiteAb, 2017
- Schieffelin, J.S., Norton, E.B., Kolls, J.K., What should define a SARS-CoV-2 “breakthrough”, The Journal of Clinical Investigation, 2021
- Schlake, T., Thess, A., Fotin-Mleczek, M. and Kallen, J., Developing mRNA-vaccine technologies, RNA biologies, National Library of Medecine
- Schoenmaker, L., Witzigmann, D., A. Kulkarni, J., Verbeke, V., Kersten, G., Jiskoot, W. and Crommelin, D.J.A., mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability, International Journal of Pharmaceutics, 2021
- Segal, E., New Vaccine Supply And Distribution Problems Slow Fight Against Covid – And Provide More Crisis Management Lessons, Forbes, 2021
- Sinovac, 20-F Annual report 2021, filed at SEC, 29 April 2022
- Storz, U., The Covid 19 vaccine patent race, 2022
- Storz, U., The patent maze of COVID 19 vaccines, Expert Opinion on Therapeutic Patents, 2021
- Taylor, M. and Respaut, R., ‘Exclusive: Moderna Spars with US Scientists over COVID-19 Vaccine Trials’, Reuters, 7 July 2020.
- Terry, M., With Supply Lagging, COVID-19 Vaccine Manufacturers Pledge to Ramp Up, Biospace, 2021
- The real-time billionaires list, Forbes, 2022
- Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: What manufacturing activities are involved and why are they so difficult? 2021
- Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: Procuring lipids and assembling lipid nanoparticles, 2021 Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: Dose selection, stability, and optimization of mRNA based COVID vaccines, 2021
- UNICEF, COVID-19 market dashboard, https://www.unicef.org/supply/COVID-19-market-dashboard , all accessed 21 may 2023.
- Weise, E. and Weintraub, K., A COVID-19 Vaccine Life Cycle: From DNA to Doses, USA Today, 7 February 2021.
- World Health Organization (WHO): Coronavirus disease (COVID-19) pandemic
- World Trade Organization, Ministerial Conference, Twelfth Session, Geneva, June 12-15, 2022
Yu, M.Z., Wang, N.N., Zhu, J.Q. and Lin, Y.X., The clinical progress and challenges of mRNA vaccines, Wiley Periodicals LLC., 2023
[1] Farmaceutico e Covid-19: Alcuni fatti stilizzati, Andrea Montanino, Simona Camerano, Alberto Carriero, Angela Cipollone, Roberto Giuzio, CDP, 2020.
[2] Farmindustria, Indicatori farmaceutici, 2022
[3] Farmindustria, Indicatori farmaceutici, 2022
[4] World Health Organization (WHO): Coronavirus disease (COVID-19) pandemic
[5] Ozili, P.K.; Arun, T. Spillover of COVID-19: Impact on the global economy, SSRN Electron. J. 2020
[6] Maffioli, E.M. In che modo il mondo sta rispondendo alla nuova malattia da coronavirus (COVID-19) rispetto all’epidemia di Ebola dell’Africa occidentale del 2014? L’importanza della Cina come attore nell’economia globale, Am. J. Trop. Med. Hyg., 2020
[7] Aljadir, A.; Alnemsh, M. Exploration of the COVID-19 pandemic in relation to the healthcare industry Supply Chain 2020
[8] Hill, A.; Wang, J.; Levi, J.; Heath, K.; Fortunak, J. Minimum costs to manufacture new treatments for COVID-19, Journal of Virus Eradication, 2020
[9] Kretchy, I.A.; Asiedu-Danso, M.; Kretchy, J.-P. Medication management and adherence during the COVID19 pandemic: Perspectives and experiences from low-and middle-income countries. Research in Social and Administrative Pharmacy 2020
[10] Almurisi, S.H., Khalidi, D.A., AL-Japairai, K.A., Mahmood, S., Chilakamarry, C.R., Kadiyala, C.B.N. and Mohananaidu, K., Impact of COVID 19 Pandemic Crisis on the Health System and Pharmaceutical Industry, Researchgate, 2021
[11] Randewich, N., Coronavirus, oil collapse erases $5 trillion from U.S. stocks, Reuters, 2020
[12] Gilles, C., Greeley, B. and Arnold, M., Financial Times: Global recession already here, say top economists, 2020
[13] Martin, E., Bloomberg, Coronavirus economic impact ‘will be severe,’ at least as bad as Great Recession, says IMF, Fortune, 2020
[14] Istituto Superiore Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/VacciniCosaSono
[15] Istituto Superiore di Sanità, epidemiology for public health, vaccines and immunisation https://www.epicentro.iss.it/vaccini/
[16] https://www.salute.gov.it/portale/home.html
[17] Decreto legge 7 giugno 2017, n. 73 https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=59548
[18] Legge 31 luglio 2017 , n. 119 https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=60201
[19] Agenzia Italiana del Farmaco (AIFA), Covid-19, Vaccines https://www.aifa.gov.it/vaccini-covid-19
[20] Pardi, N., Hogan, M., Porter, F. & Weissman, D. – mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery 17, 261-279, 2018.
[21] Garde, D. & Saltzman, J. – The story of mRNA: How a once-dismissed idea became a leading technology in the Covid vaccine race. Stat, 2020
[22] Schlake, T., Thess, A., Fotin-Mleczek, M. and Kallen, J., Developing mRNA-vaccine technologies, RNA biologies, National Library of Medecine
[23] Rosa, S.S., Prazeres, D.M.F., Azevedo, A.M. and Marques, M.P.C. (2021), mRNA vaccines manufacturing: challenges and bottlenecks. https://www.sciencedirect.com/science/article/pii/S0264410X21003194?via%3Dihub
[24] Istituto di ricerche farmacologiche Mario Negri IRCCS, mRNA vaccines https://www.marionegri.it/magazine/vaccini-a-mrna
[25] Biontech.de, The role of mRNA from the vaccine https://impfung.biontech.de/it/impfung/mechanism-of-mrna.html
[26] Hopkins, J.S., Eastwood, J. and Moriarty, D., mRNA Covid-19 Vaccines Are Fast to Make, but Hard to Scale: complex steps of new type of vaccine create production bottlenecks, The Wall Street Journal, 2021
[27] Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: What manufacturing activities are involved and why are they so difficult? 2021
[28] Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: Procuring lipids and assembling lipid nanoparticles, 2021
[29] Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: Procuring lipids and assembling lipid nanoparticles, 2021
[30] Sadler, R., Who are the top suppliers in the Lipid market?, CiteAb, 2017
[31] Heilweil, R., The key ingredient that could hold back vaccine manufacturing, Vox, 2021
[32] Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: Procuring lipids and assembling lipid nanoparticles, 2021
[33] Roces, C.B., Lou, G., Jain, N., Abraham, S., Thomas, A., Halbert, G.W. and Perrie, Y., Manufacturing Considerations for the Development of Lipid Nanoparticles Using Microfluidics, MDPI Pharmaceutics, 2020
[34] Tulum, O., Lazonick, W. and Jacobson, K., Scaling of COVID vaccine manufacturing: Dose selection, stability, and optimization of mRNA based COVID vaccines, 2021
[35] LeClair, D.A., Cranston, E.D., Lichty, B.D., Xing, Z., Thompson, M.R., Consecutive spray drying to produce coated dry powder vaccines suitable for oral administration, ACS Biomater Sci Eng. 2018
[36] AboulFotouh, K., Cui, Z. and Williams III, R.O., Next-Generation COVID-19 Vaccines Should Take Efficiency of Distribution into Consideration, AAPS PharmSciTech. 2021
[37] Schoenmaker, L., Witzigmann, D., A. Kulkarni, J., Verbeke, V., Kersten, G., Jiskoot, W. and Crommelin, D.J.A., mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability, International Journal of Pharmaceutics, 2021
[38] European Medicines Agency, Studies for approval https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-studies-approval
[39] European Medicines Agency, human regulatory, Covid-19 https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-development-evaluation-approval-monitoring
[40] Indicative timelines for COVID-19 vaccines compared with standard vaccines, European Medicines Agency, Covid 19https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-development-evaluation-approval-monitoring
[41] COVID-19 guidance: evaluation and marketing authorisation, European Medicines Agency https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/guidance-developers-companies/covid-19-guidance-evaluation-marketing-authorisation
[42] Rutschman, A.S., The Vaccine Race in the 21st Century, 61 ARIZ. L. REV. 2019
[43] Grisoli A., La secolare ed incompiuta vicenda della brevettabilità dei farmaci, in Riv. dir. ind. 1981
[44] Arnaudo L., Pitruzzella G., La cura della concorrenza. L’industria farmaceutica tra diritti e profitti, Roma, 2019
[45] Galli, C., Il diritto della proprietà intellettuale di fronte alle sfide della pandemia, in Dir. ind., 2021
[46] Massimiliano F., Vaccini, Brevetti e Big Pharma tra profitto, sostenibilità e diritto alla salute, in Dir. ind., 2021
[47] For the purpose of this Decision, all developing country Members are eligible Members. Developing country Members with existing capacity to manufacture COVID-19 vaccines are encouraged to make a binding commitment not to avail themselves of this Decision. Such binding commitments include statements made by eligible Members to the General Council, such as those made at the General Council meeting on 10 May 2022, and will be recorded by the Council for TRIPS and will be compiled and published publicly on the WTO website. – World Trade Organization, Ministerial Conference, Twelfth Session, Geneva, June 12-15, 2022
[48] For the purpose of this Decision, it is understood that ‘subject matter of a patent’ includes ingredients and processes necessary for the manufacture of the COVID-19 vaccine – World Trade Organization, Ministerial Conference, Twelfth Session, Geneva, June 12-15, 2022
[49] Storz, U., The patent maze of COVID 19 vaccines, Expert Opinion on Therapeutic Patents, 2021
[50] Large nodes represent the relevant entities while the edges represent agreements or patents between two entities. Smaller nodes around the entities represent patents that were identified as being relevant to the underlying vaccine technology. Gaviria, M., Kilic, B. A network analysis of COVID-19 mRNA vaccine patents. Nat Biotechnol.
[51] Council for Trade-Related Aspects of Intellectual Property Rights, WHO IP/C/W/ 669, 2.10.2020
[52] World Trade Organization, Ministerial Conference, Twelfth Session, Geneva, June 12-15, 2022
[53] Storz, U., The patent maze of COVID 19 vaccines, Expert Opinion on Therapeutic Patents, 2021
[54] Cullinal, K., Wealthy Countries Urged to Share 2 Billion Excess COVID-19 Doses, as South Africa’s mRNA Hub Prepares to Make ‘Moderna-like’ Vaccine, Health Policy Watch, 2021
[55] Dolgin, E., The tangled history of m RNA vaccines, Nature, 2021
[56] Storz, U., The patent maze of COVID 19 vaccines, Expert Opinion on Therapeutic Patents, 2021
[57] Storz, U., The patent maze of COVID 19 vaccines, Expert Opinion on Therapeutic Patents, 2021
[58] Storz, U., The patent maze of COVID 19 vaccines, Expert Opinion on Therapeutic Patents, 2021
[59] Storz, U., The Covid 19 vaccine patent race, 2022
[60] Segal, E., New Vaccine Supply And Distribution Problems Slow Fight Against Covid – And Provide More Crisis Management Lessons, Forbes, 2021
[61] Dakin, J., Supply Chain Challenges Creating Hurdles to COVID-19 Vaccine Production, Pharmtech, 2021
[62] King, A., Why manufacturing Covid vaccines at scale is hard, ChemistryWorld, 2021
[63] Terry, M., With Supply Lagging, COVID-19 Vaccine Manufacturers Pledge to Ramp Up, Biospace, 2021
[64] Pancevski, B., BioNTech Recruits Rivals to Boost Covid-19 Vaccine Production, The Wall Street Journal, 2021
[65] Nicholson Price II, W., Rai, A. K., and Minssen, T., Knowledge transfer for large-scale vaccine manufacturing, Researchgate, 2020
[66] EMA, Biosimilar medicines: overview, https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview
[67] EMA, Transfer of marketing authorisation: questions and answers, Transfer of marketing authorisation: questions and answers | European Medicines Agency (europa.eu)
[68] Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use – First Single-Shot Vaccine in Fight Against Global Pandemic, 2021 Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use | Johnson & Johnson (jnj.com)
[69] COVAX Working Group on delivery costs, Costs of delivering COVID-19 vaccine in 92 AMC countries, section 5.2, table 9, 2021, Costs of delivering COVID-19 vaccine in 92 AMC countries (who.int)
[70] Moderna, Patents and Intellectual Property, Moderna’s Patents and pending patent applications (modernatx.com)
[71] Gaviria, M. and Kilic, B., BioNTech and Pfizer’s BNT162 Vaccine Patent Landscape, Public Citizen, 2020
[72] Dolgin, E., Unlocking the potential of vaccines built on messenger RNA. The technology could help to boost immunity against cancer, influenza and much more, Nature, 2019
[73] Rutschman, A.S., IP Preparedness for Outbreak Diseases, UCLA L. REV., 2018
[74] European Commission, Safe Covid-19 vaccines for Europeans, https://commission.europa.eu/strategy-and-policy/coronavirus-response/safe-covid-19-vaccines-europeans_en
[75] European Commission, Safe Covid-19 vaccines for Europeans, https://commission.europa.eu/strategy-and-policy/coronavirus-response/safe-covid-19-vaccines-europeans_en
[76] Weise, E. and Weintraub, K., A COVID-19 Vaccine Life Cycle: From DNA to Doses, USA Today, 7 February 2021.
[77] Istituto Superiore di Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/covid-19-vaccino-pfizer-biontech
[78] Taylor, M. and Respaut, R., ‘Exclusive: Moderna Spars with US Scientists over COVID-19 Vaccine Trials’, Reuters, 7 July 2020.
[79] Miller, J., ‘Moderna Vaccine to Criss-Cross Continent before Europeans Get Shots’, Reuters, January 6, 2021
[80] Istituto Superiore di Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/covid-19-vaccino-moderna
[81] Istituto Superiore di Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/covid-19-vaccino-astrazeneca
[82] Istituto Superiore di Sanità, Vaccini e vaccinazioni, https://www.epicentro.iss.it/vaccini/covid-19-vaccino-janssen
[83] Ledley, F. D., McCoy, S. S., Vaughan, G., Cleary, E. G., Profitability of large pharmaceutical companies compared with other large public companies, JAMA, 2020
[84] Fernandez, R. and Klinge, T. J., The financialisation of Big Pharma, SOMO, 2020
[85] Advanced Gene-based Vaccines along with the Advent of the Pandemic boomed the Vaccine Market: Analysis by FMI for 30+ countries, FutureMarketInsights, 2023 https://www.futuremarketinsights.com/reports/vaccines-market
[86] de Haan, E. and ten Kate A., Pharma’s Pandemic Profits, Pharma profits from COVID-19 vaccines, SOMO, 2023
[87] Moderna, 10-K Annual report 2021, filed at SEC, 25 February 2022
[88] Moderna, 10-Q report third quarter 2022, filed at SEC, 3 November 2022
[89] Pfizer, 10-Q report third quarter 2022, filed at SEC, 9 November 2022
[90] BioNTech, 6-K report third quarter 2022, filed at SEC, 7 November 2022
[91] Annual and quarterly reports of the seven companies. Biomedical Advanced Research and Development Authority (BARDA), COVID-19 Medical Countermeasure Portfolio
[92] BioNTech, 20-F Annual report 2021, filed at SEC, March 2022, page 206 and Pfizer, 20-F Annual report 2021, filed at SEC, February 2022.
[93] German Federal Ministry of Education and Research, Coronavirus: What the BMBF is doing, 2020
[94] Moderna, 10-Q report third quarter 2022, filed at SEC, 3 November 2022
[95] Biomedical Advanced Research and Development Authority (BARDA), COVID-19 Medical Countermeasure Portfolio
[96]CEPI statement: CEO welcomes Emergency Use Listing for NVX-CoV2373, CEPI, 17 December 2021
[97] Novavax Inc., Annual report 2021 including 10-K form, filed at SEC, March 2022
[98] Novavax, Investor presentation, January 2023, https://novavax.widen.net/s/bsldbcxbxh/novavax_corporate_investor_ deck_2023-01-09
[99] AstraZeneca, Annual Report and Form 20-F Information for 2020 and AstraZeneca, Annual Report and Form 20-F Information for 2021
[100] Biomedical Advanced Research and Development Authority (BARDA), COVID-19 Medical Countermeasure Portfolio
[101] De Haan, E. and ten Kate, A., Pharma’s Pandemic Profits, Pharma profits from COVID-19 vaccines, SOMO, 2023
[102] UNICEF, COVID-19 market dashboard, https://www.unicef.org/supply/COVID-19-market-dashboard , all accessed 21 may 2023.
[103] Governments spent at least €93bn on COVID-19 vaccines and therapeutics during the last 11 months, Business wire, 11 January 2021
[104] Geneva Graduate Institute, Global Health Centre, COVID-19 Vaccines R&D Investments, 2021
[105] Pfizer, 10-Q report third quarter 2022, filed at SEC, 9 November 2022
[106] BioNTech, Share Repurchase Program, https://investors.biontech.de/share-repurchase
[107] Moderna reports third quarter 2022 financial results and provides business updates, Moderna, 3 November 2022 and Moderna 2Q 2022 Earnings Call, Moderna, 3 August 2022
[108] Sinovac, 20-F Annual report 2021, filed at SEC, 29 April 2022
[109] Pfizer, 20-F Annual report 2021, filed at SEC, February 2022
[110] Pfizer, 8-K report earnings release 2022, filed at SEC, 31 January 2023
[111] Arvinas and Pfizer Announce Global Collaboration to Develop and Commercialize PROTAC® Protein Degrader ARV-471, Pfizer, 22 July 2021
[112] Pfizer and Beam enter exclusive multi-target research collaboration to advance novel in vivo base editing programs for a range of rare diseases, Pfizer, 10 January 2022
[113] Moderna, 10-Q report third quarter 2022, filed at SEC, 3 November 2022
[114] Moderna, ESG Day, 10 November 2022
[115] BioNTech, 20-F Annual report 2021, filed at SEC, March 2022
[116] Breakthrough technologies across four different drug classes to revolutionize medicine, BioNTech,
[117] Pfizer, Third Quarter 2022 earnings teleconference, 1 November 2022
[118] Pfizer, Proxy Statement for 2022 Annual Meeting of Shareholders, Summary Compensation Table, 28 April 2022,
[119] The real-time billionaires list, Forbes, 2022
[120] Biopharma and Medtech Review 2022, Evaluate Vantage, 2023
[121] Biopharma and Medtech Review 2022, Evaluate Pharma, 2023. Companies are assigned these groups based on year-end 2021 market caps, and remain in the same group for the rest of the year.
[122] Biopharma and Medtech Review 2022, Evaluate Pharma, 2023. Companies are assigned these groups based on year-end 2021 market caps, and remain in the same group for the rest of the year.
[123] Biopharma and Medtech Review 2022, Evaluate Vantage, 2023
[124] Biopharma and Medtech Review 2022, Evaluate Pharma, 2023
[125] Biopharma and Medtech Review 2022, Evaluate, 2023. This analysis of data concerns IPOs of pure-play drug developers only; secondary listings are not included.
[126] Biopharma and Medtech Review 2022, Evaluate, 2023
[127] Biopharma and Medtech Review 2022, Evaluate Pharma, 2023
[128] World-famous Italian microbiologist, Scientific Director of the Biotecnopolo Foundation of Siena, of which the National Anti-Pandemic Centre (CNAP) is also a member, scientific coordinator of the MAD Lab at Toscana Life Sciences in Siena, and winner of several awards in the medical and vaccination field, interviewed by candidate Alice Pizziconi, on June 6, 2023.
[129] Rusnock, A., Catching cowpox: the early spread of smallpox vaccination, 1798–1810, National Library of Medicine, Bull Hist Med., 2009
[130] Yu, M.Z., Wang, N.N., Zhu, J.Q. and Lin, Y.X., The clinical progress and challenges of mRNA vaccines, Wiley Periodicals LLC., 2023
[131] Harding, A.T., Heaton, N.S., Efforts to improve the seasonal influenza vaccine, National Library of Medicine, Vaccines, 2018
[132] Armbruster, N., Jasny, E. and Petsch, B., Advances in RNA vaccines for preventive
indications: a case study of A vaccine against rabies, MDPI open access journals, 2019
[133] Diamond, D.J., Rosa, C.L., Chiuppesi, F., Contreras, H., Dadwal, S., Wussow, F., Bautista, S., Nakamura, R. and Zaia, J.A., A fifty-year odyssey: prospects for a cytomegalovirus vaccine in transplant and congenital infection, Expert Review of vaccines, Volume 17, 2018
[134] Kose, N., Fox, J.M., Sapparapu, G., Bombardi, R., Ennekoon, T.R.N., Silva, A.D.D., Elbashir, S.M., Theisen, M.A., Narayanan, E.H., Ciaramella, G., Himansu, S., Diamond, M.S. and Crowe, J.E., A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection, Scopus, Science Immunology, 2019
[135] Abdel-Hakeem, M.S., Shoukry N.H., Protective immunity against hepatitis C: many shades of gray, National Library of Medicine, Front. Immunol., 2014
[136] Lopez Angel, C.J., Tomaras, G.D., Bringing the path toward an HIV-1 vaccine into focus, National Library of Medicine, PLOS Pathogens, 2020
[137] Mallory, K.L., Taylor, J.A., Zou, X., Waghela, I.N., Schneider, C.G., et al., Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice, National Library of Medicine, NPJ Vaccines, 2021
[138] Moriya, T., Kitagawa, K., Hayakawa, Y., Hemmi, H., Kaisho, T., Ueha, S., Ikebuchi, R., Yasuda, I., Nakanishi, Y., Honda, T., Matsushima, K., Kabashima, K., Ueda, M., Kusumoto, Y., Chtanova, T., Tomura, M., Immunogenic tumor cell death promotes dendritic cell migration and inhibits tumor growth via enhanced T cell immunity, iScience volume 24, 2021
[139] Li, L., Goedegebuure, S.P., Gillanders, W., Cancer vaccines: shared tumor antigens return to the spotlight, Signal Transduction Targeted Therapies 5, article n. 251, 2020
[140] Yu, M.Z., Wang, N.N., Zhu, J.Q. and Lin, Y.X., The clinical progress and challenges of mRNA vaccines, Wiley Periodicals LLC., 2023
[141] World-famous Italian microbiologist, Scientific Director of the Biotecnopolo Foundation of Siena, of which the National Anti-Pandemic Centre (CNAP) is also a member, scientific coordinator of the MAD Lab at Toscana Life Sciences in Siena, and winner of several awards in the medical and vaccination field, interviewed by candidate Alice Pizziconi, on June 6 2023.
[142] Rappuoli, R., Personal communication, June 6, 2023
[143] Rappuoli, R., Personal communication, June 6, 2023
[144] Rappuoli, R., Personal communication, June 6, 2023
[145] Rappuoli, R., Personal communication, June 6, 2023
[146] Rappuoli, R., Personal communication, June 6, 2023
[147] Rappuoli, R., Personal communication, June 6, 2023
[148] Schieffelin, J.S., Norton, E.B., Kolls, J.K., What should define a SARS-CoV-2 “breakthrough”, The Journal of Clinical Investigation, 2021
[149] Duffy, S., Why are RNA virus mutation rates so damn high?, PLOS Biology, 2018
[150] Beatty, G.L., Gladney, W.L., Immune escape mechanisms as a guide for cancer immunotherapy, National Library of Medicine, Clin. Cancer Res., 2015
[151] Rosenthal, R., Cadieux, E.L., Salgado, R., Bakir, M.A., Moore, D.A., et al., Neoantigen-directed immune escape in lung cancer evolution, National Library of Medicine, Nature, 2019
[152] Rosa, S.S., Prazeres, D.M.F., Azevedo, A.M., Marques, M.P.C., mRNA vaccines manufacturing: challenges and bottlenecks, National Library of Medicine, Vaccine, 2021
[153] Liu, T., Liang, Y., Huang, L., Development and delivery systems of mRNA vaccines, National Library of Medicine, Frontiers in Bioengineering and Biotechnology, 2021
[154] Rosa, S.S., Prazeres, D.M.F., Azevedo, A.M., Marques, M.P.C., mRNA vaccines manufacturing: Challenges and bottlenecks, National Library of Medicine, Vaccine, 2021
[155] Rappuoli, R., Personal communication, June 6, 2023
[156] Rappuoli, R., Personal communication, June 6, 2023
[157] Rappuoli, R., Personal communication, June 6, 2023